TOWARDS DEVELOPMENT OF BAYLIS-HILLMAN REACTION
ORIGIN
Since my post-graduation (M. Sc.) days (1970-1972) at Department of Chemistry, Banaras Hindu University (BHU) I was
fascinated by the Diels-Alder reaction because of its high and diverse levels of applications in organic synthesis.
During that time we had excellent teachers who had tremendous influence on my thinking about organic chemistry. Professor
Gurbakhsh Singh (who did Ph. D. at Harvard with Professor R. B. Woodward) was one such teacher whose brilliant teachings
clearly indicated that there is a need to discover/uncover many more new reactions and synthetic strategies, although
there are many name and unnamed reactions, to meet the challenges arising from continuous developments. It was also very
clear from his thought provoking lectures that discovering/ developing new useful organic reaction(s) will not only be
extremely difficult but also be the most challenging research endeavors that need several years of hard work and
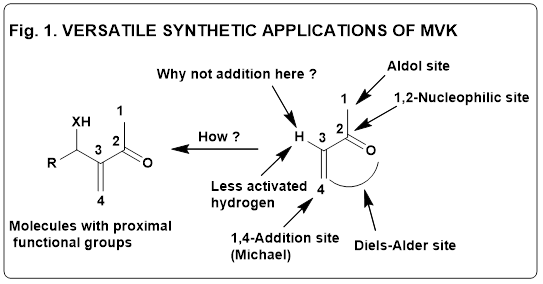
dedicated efforts. During that time, the molecule which had great impact on my chemistry understanding was methyl vinyl
ketone (MVK) (Fig. 1) because of its high reactivity and versatility (aldol reaction at C-1; 1,2-addition at C-2; Michael
reaction at C-4; and as diene for Diels-Alder reaction). One question used to bother me: Why there was no reaction known
at C-3 of MVK as shown in Fig. 2 ? One day I asked Professor Gurbakhsh Singh the same question after his lecture. He smiled
and told me that this aspect was not studied till then and performing any reaction at C-3 of MVK would, in fact, be
challenging and useful endeavor. It was indeed a very interesting answer.
After my M. Sc., I joined the research group of Professor Gurbakhsh Singh for Ph. D. program at BHU. During those days I
also had an opportunity of having excellent senior colleagues. In the beginning of my Ph. D. days, I started looking into
patents through Chemical Abstracts based on the suggestion of a senior colleague with a view to originate ideas for
research work. At that time, I came across a brilliant and highly promising patent by Baylis and Hillman (1972)
(I used to write interesting reactions in my library note book and thus this patent information was noted down).1
This patent describes the coupling reaction between α-position of acrylates / acrylonitrile/ methyl vinyl ketone/ acrylamide
with aldehydes under the influence of a tertiary amine to provide multifunctional molecules. This patent impressed me very
much. I started keeping track of any research work in the literature related to this patent. After obtaining my Ph. D.
degree I joined the research group of Professor Herbert C. Brown at Purdue University for three years, that is, during 1980-
1983 as a post doctoral fellow.
I was surprised to note that no publication appeared in the literature having any relation with this fascinating patent
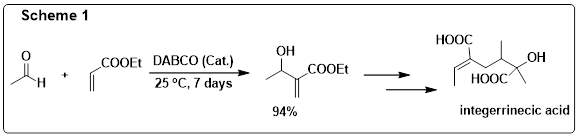
for almost a decade (during 1972-1981). I saw the first publication in the year 1982 by Drewes and Emslie from South Africa,
on the application of patent of Baylis and Hillman.2 This paper describes an interesting DABCO catalyzed reaction between
ethyl acrylate and acetaldehyde to provide the corresponding adduct which was subsequently transformed into integerrinecic
acid (Scheme 1).2
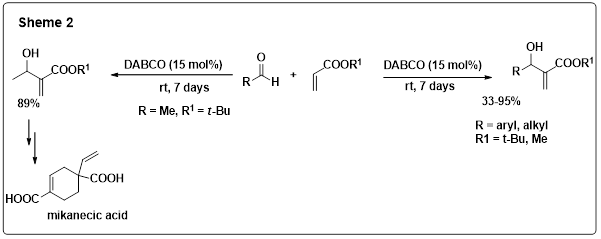
In the subsequent year (1983), Hoffmann and Rabe (from Germany) published two back to back papers on the coupling of methyl and t-butyl acrylates with aldehydes using DABCO as catalyst.3,4 One such resulting adduct thus obtained via the reaction of acetaldehyde with t-butyl acrylate was elegantly used for the synthesis of mikanecic acid (Scheme 2).3
After being fascinated by Baylis and Hillman patent, I checked the literature thoroughly to see whether there was anything known before this. I noticed three interesting reports: (1) A patent by Rauhut and Currier on trialkyl-phosphine catalyzed dimerization of alkyl acrylates to provide dialkyl 2-methylene-glutarate derivatives in the year 1963 (one example is given in Eq. 1),5 (2) Two brilliant publications in the year 1968 by Morita and coworkers who reported tricyclohexylphosphine catalyzed reaction between aldehydes and acrylates to provide 2-methylene-3-hydroxyalkanoates. (Eq. 2).6,7

- a) A. B. Baylis, M. E. D. Hillman, German patent 2155113, 1972; Chem. Abstr. 1972, 77, 34174q; b) M. E. D. Hillman, A. B. Baylis, U.S. Patent 3,743, 669, 1973.
- S. E. Drewes, N. D. Emslie, J. Chem. Soc.,Perkin Trans. I 1982, 2079.
- H. M. R. Hoffmann, J. Rabe, Angew. Chem. Int. Ed. Engl. 1983, 22, 795.
- J. Rabe, H.M. R. Hoffmann, Angew Chem. Int. Ed. Engl.1983, 22, 796.
- M. M. Rauhut, H. Currier, American Cyanamide Co. U. S. Patent, 1963, 3074999; Chem. Abstr. 1963, 58, 11224a.
- K. Morita, Z. Suzuki, H. Hirose, Bull. Chem. Soc. Jpn. 1968, 41, 2815.
- K. Morita, T. Kobayashi, Bull. Chem. Soc. Jpn. 1969, 42, 2732.
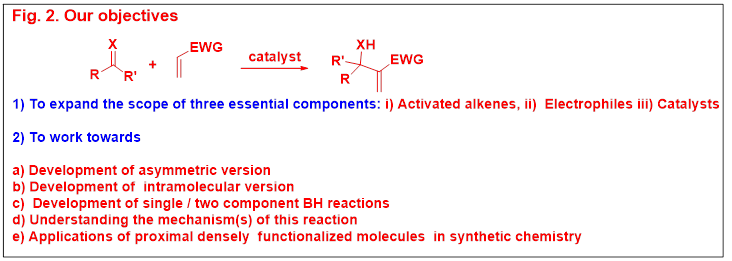
OUR VISION AND AIM
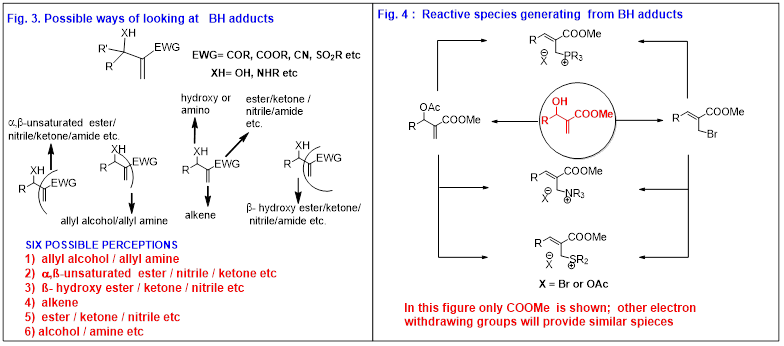
Based on the above-mentioned Baylis-Hillman patent (actually buried in the literature) information and a few other related reports, we envisioned in the year 1984 that the patent by Baylis and Hillman will not only have a great potential as a novel three component C-C bond forming reaction (see Fig. 2) but also create challenging opportunities toward discovering new directions in organic chemistry in the years to come. Having envisioned the great potential of Baylis-Hillman patent as shown in Fig.2 we surmised that this reaction would address and fit best into the philosophy of our research. This reaction would provide molecules with three proximal functional groups via atom-economical formation of C-C-bond. These molecules with three proximal functional groups can be viewed in six different possible ways (Fig.3) and thus chemistry of these molecules can be designed and executed. In addition, this class of molecules can serve as a source of various reactive species which will offer new avenues in synthetic and mechanistic chemistry (Fig.4). On the basis of the above-mentioned insight we also believed that this reaction will prove to be as useful and popular as the well known Diels-Alder reaction. Thus our aim is to work in this direction, and make this reaction if possible, more useful and popular than the Diels-Alder reaction.