Publication Details:
Title: Kinetic resolution of sulfur-stereogenic sulfoximines by Pd(II)–MPAA catalyzed C–H arylation and olefination
| Author(s) | Kallol Mukherjee, Nicolas Grimblat, Somratan Sau, Koushik Ghosh, Majji Shankar, Vincent Gandon and Akhila K. Sahoo* : Chem. Sci., 2021, 12, 14863-14870. |
|---|---|
| Journal Name | Chemical science |
| journal image URL | http://chemistry.uohyd.ac.in/~aks/wp-content/uploads/chemicalSc.jpg |
More Details:
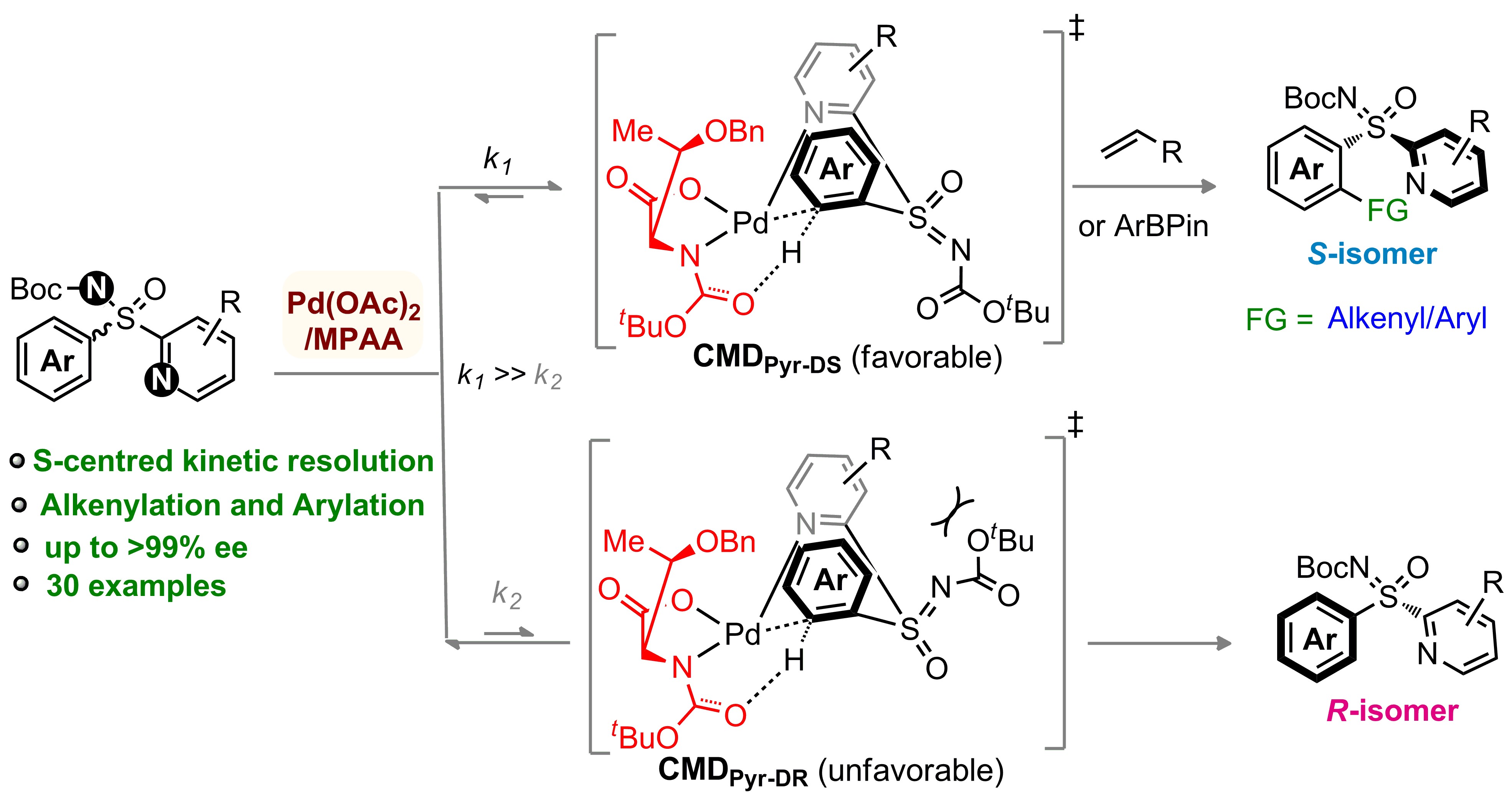
A direct Pd(II)-catalyzed kinetic resolution of heteroaryl-enabled sulfoximines through an ortho-C–H alkenylation/arylation of arenes has been developed. The coordination of the sulfoximine pyridyl-motif and the chiral amino acid MPAA ligand to the Pd(II)-catalyst controls the enantio-discriminating C(aryl)–H activation. This method provides access to a wide range of enantiomerically enriched unreacted aryl-pyridyl-sulfoximine precursors and C(aryl)–H alkenylation/arylation products in good yields with high enantioselectivity (up to >99% ee), and selectivity factor up to >200. The coordination preference of the directing group, ligand effect, geometry constraints, and the transient six-membered concerted-metalation–deprotonation species dictate the stereoselectivity; DFT studies validate this hypothesis.
https://pubs.rsc.org/en/content/articlelanding/2021/SC/D1SC04299H