Publication Details:
Title: Cationic-Palladium Catalyzed Regio- and Stereoselective syn-1,2-Dicarbofunctionalization of Unsymmetrical Internal Alkynes
| Author(s) | Shubham Dutta, Shashank Shandilya, Shengwen Yang, Manash Protim Gogoi, Vincent Gandon, and Akhila K. Sahoo* : Nat. Commun., 2022, 13, 1360. |
|---|---|
| Journal Name | Nature Communications |
| journal image URL | http://chemistry.uohyd.ac.in/~aks/wp-content/uploads/Nature-Communications-logo.jpg |
More Details:
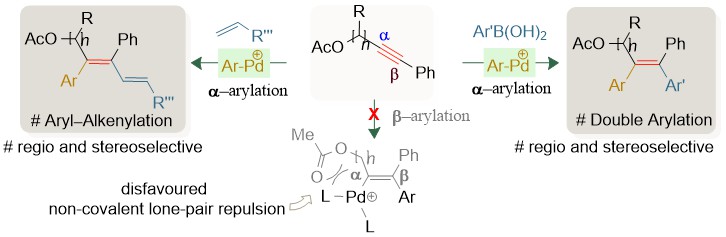
π-Extended tetrasubstituted olefins are widely found motifs in natural products, leading drugs, and agrochemicals. Thus, development of modular strategies for the synthesis of complex all-carbon-substituted olefins always draws attention. The difunctionalization of unsymmetrical alkynes is an attractive approach but it has remained faced with regioselectivity issues. Here we report the discovery of a regio- and stereoselective syn-1,2-dicarbofunctionalization of unsymmetrical internal alkynes. A cationic Pd-catalyzed three-component coupling of aryl diazonium salts, aryl boronic acids (or olefins) and yne-acetates enables access to all-carbon substituted unsymmetrical olefins. The transformation features broad scope with labile functional group tolerance, building broad chemical space of structural diversity (94 molecules). The value of this synthetic method is demonstrated by the direct transformation of natural products and drug candidates containing yne-acetates, to enable highly substituted structurally complex allyl acetate analogues of biologically important compounds. Synthetic versatility of the carboxylate bearing highly-substituted olefins is also presented. The reaction outcome is attributed to the in-situ formation of stabilized cationic aryl-Pd species, which regulates regioselective aryl-palladation of unsymmetrical yne-acetates. Control experiments reveal the synergy between the carboxylate protecting group and the cationic Pd-intermediate in the regioselectivity and reaction productivity; density functional theory (DFT) studies rationalize the selectivity of the reaction.