‘Ca’talysis Lab
Metal-Catalyzed Functionalization of Alkynes/Alkynols
Our Group works broadly in Synthetic Organic Chemistry, Particularly towards the development of new Metal-Catalyzed Synthetic Methodologies. The core idea is to develop reactions from simple/easily /readily available materials to make molecular complexity and small molecules of potential interest. We use the in situ formed allenes, C-acylimines, propargyl alcohols, and push-pull enamines as the key building blocks for the synthesis of various privileged molecules. These methods may involve a green chemical approach, such as cascade/one-pot multicomponent methods, sometimes catalyst-free/solvent-free methods. The synthesized compounds would be tested for biological activity, and we also study the preliminary photophysical properties of selected molecules to see their utility in material science.
The key research topics are:
1. Calcium-Catalysis (Emerging Green Catalysis)
We are one of the leading research groups in exploring Calcium salts as an alternative Lewis acid catalyst (alternative to some of the transition metal-catalysis) for Organic Reactions. Owing to its high abundance, less toxic nature, biodegradability, moisture tolerance, We have been successfully using Calcium catalysts for the development of various reactions. It is now called a sustainable catalyst.
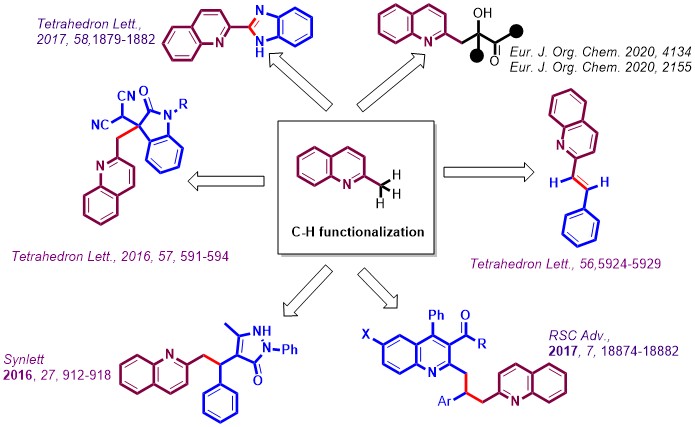
2. C-H Functionalization
Owing to the potential interest in quinoline scaffolds, C-H Functionalization of 2-Methylquinolines using simple reaction conditions has been developed by us. We were successful in utilizing Ca-catalysts for this purpose. Some of the preliminary results are shown here
3. Donor-Acceptor Cyclopropanes
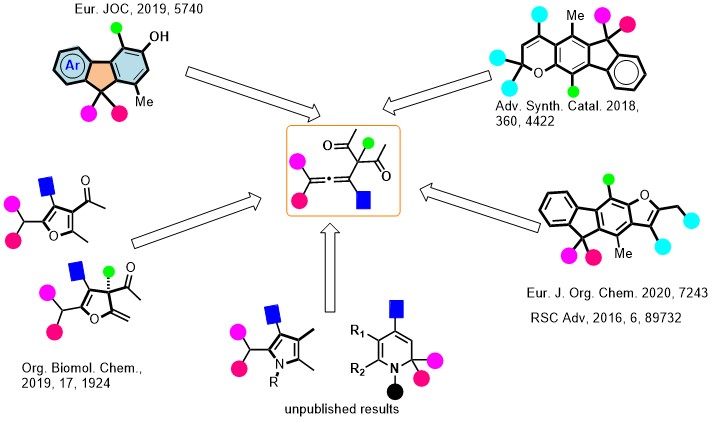
4. Allene Chemistry
We have developed a Calcium-catalyzed, in situ preparation of fully substituted allenes, and their domino cyclization reactions to furnish New chemical entities. The representative examples are shown here
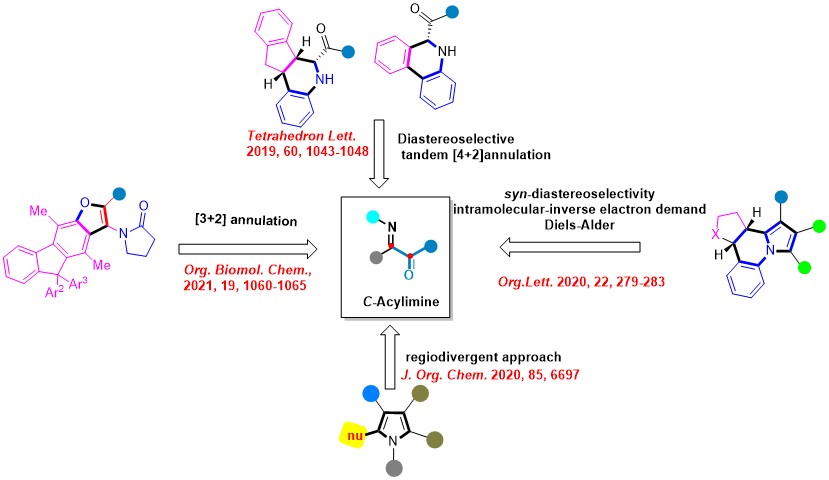
5. Chemistry of a-Iminoketones
In recent years, we have started utilizing the in situ formed C-Acylimines as the key building blocks for various organic transformations. Some of the selected examples are listed here
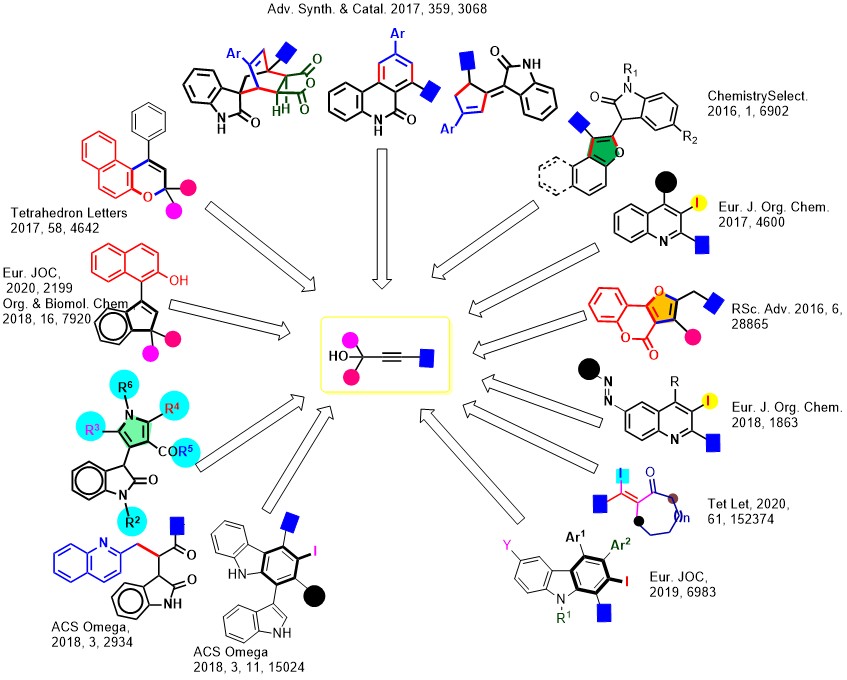
6. Functionalization of Alkynes/Alkynols
This is one of the major research areas of our group, we have engineered the chemical reactivity of propargyl alcohols in various controlled, regioselective reactions. We have extended this, to Enyne-Cycloisomerization, Iodo-Cyclizations, Ring-Rearrangements, and benzannulation reactions.