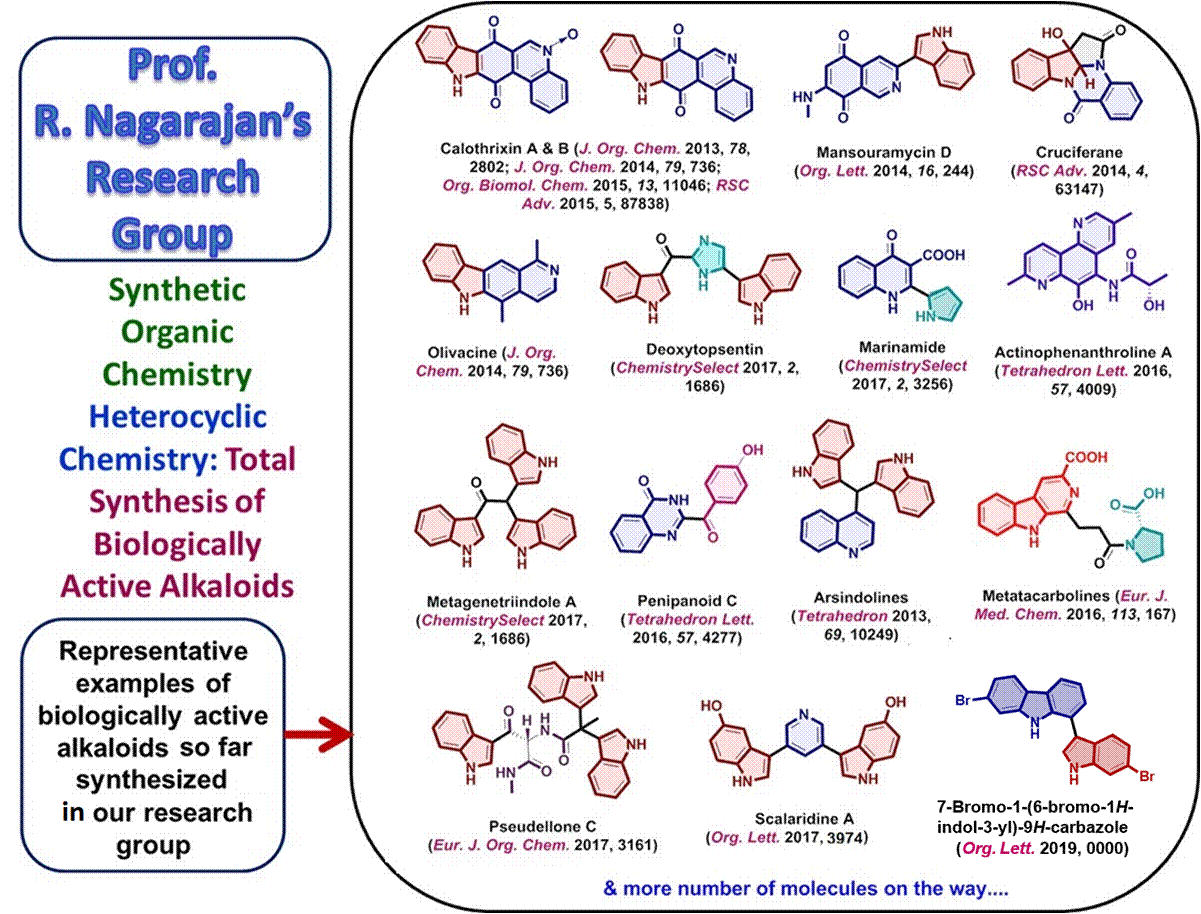
Synthetic
Organic Chemistry
Heterocyclic Chemistry - Total Synthesis
of Alkaloids
Some
examples of synthetic methodology developed for biologically active alkaloids
in my group:
A Formal Synthesis of Lavendamycin Methyl
Ester and Nitramarine via Povarov
Approach
S. Ramesh and R. Nagarajan, J. Org. Chem., 2013, 78,
545.
Total
synthesis of anti-malarial alkaloids Calothrixin A & B:
N. Ramkumar and R.
Nagarajan, J. Org. Chem., 2013, 78, 2802;
N. Ramkumar and R.
Nagarajan, J. Org. Chem., 2014, 79, 736;
N. Ramkumar and R.
Nagarajan, Org. Biomol. Chem., 2015, 13,
11046;
N. Ramkumar and R.
Nagarajan, RSC Advances, 2015,
5, 87838.
First synthesis of bis(indolyl)methane alkaloids arsindoline A and B using low melting mixture
R. R. Jella
and R. Nagarajan, Tetrahedron, 2013, 69, 10249.
First
total synthesis of cytotoxic alkaloid Mansouramycin D
employing imino annulation reaction as the key step
K.
S. Prakash and R. Nagarajan, Org. Lett., 2014,
72, 3573.
The first synthesis of a bisindole alkaloid, Pseudellone C using protecting‐group free, economic
and commercially available starting materials was achieved in 44 %
overall yield.
P. P. Sathieshkumar, P. Latha
and R. Nagarajan, Eur. J. Org. Chem.,
2017, 3161.
A
ligand-free, copper catalyzed Suzuki-Miyaura coupling
route to synthesis of bisindole alkaloid Scalaridine A
G.
Ranjani and R. Nagarajan, Org. Lett., 2017, 19, 3974.
First total synthesis of naturally rare dibromo
1-(indol-3-yl)carbazole
alkaloid in two steps
G. Ranjani
and R. Nagarajan, Org. Lett., 2019,
21, 675.
Metal-Free Cross-Dehydrogenative Coupling
approach for the Synthesis of 1-indolyltetrahydro-β-carbolines with an
application to the total synthesis of Eudistomin U, Isoeudistomin U and 19-Bromoisoeudistomin U
G. Ranjani and
R. Nagarajan, Chem. Commun., 2021, 57, 757.
First total synthesis of (±)-Oxoaplysinopsin B via Cascade approach
P. P. Sathieshkumar, M. D. A. Saibabu and R. Nagarajan, J. Org. Chem., 2021, 86, 3730.