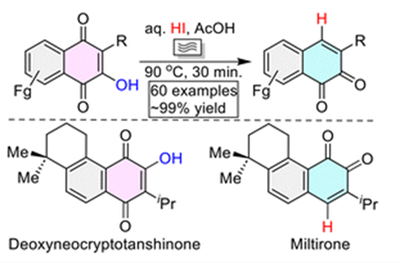
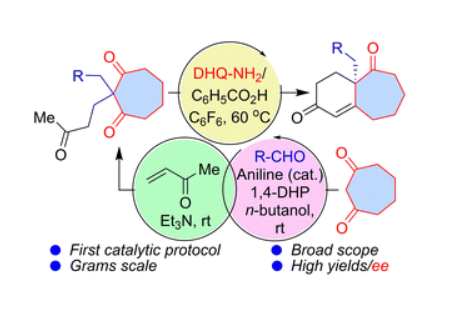
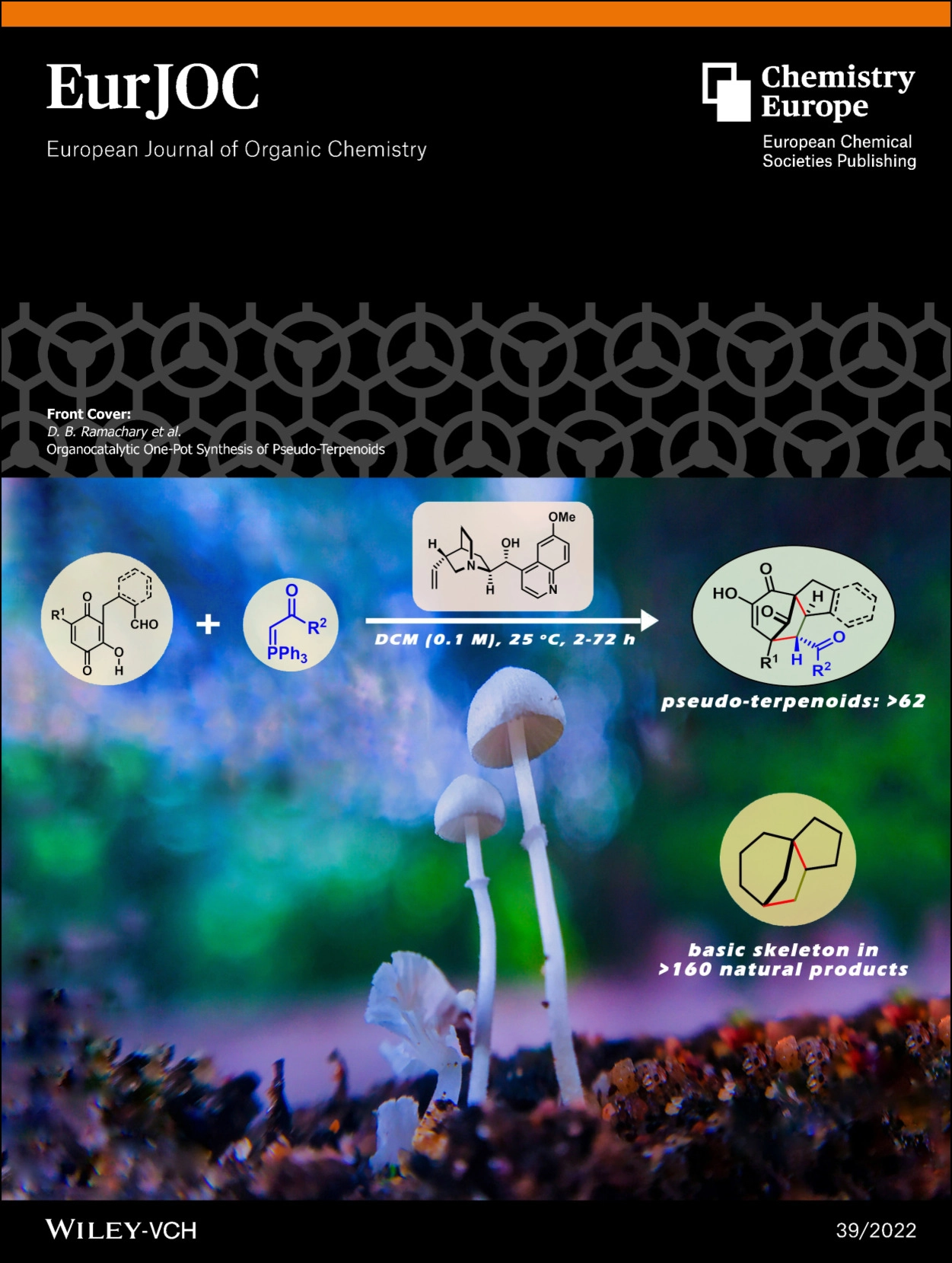
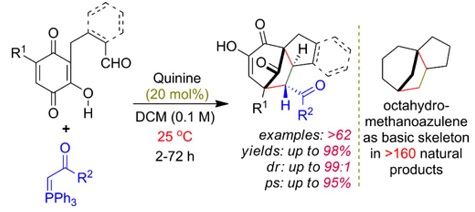
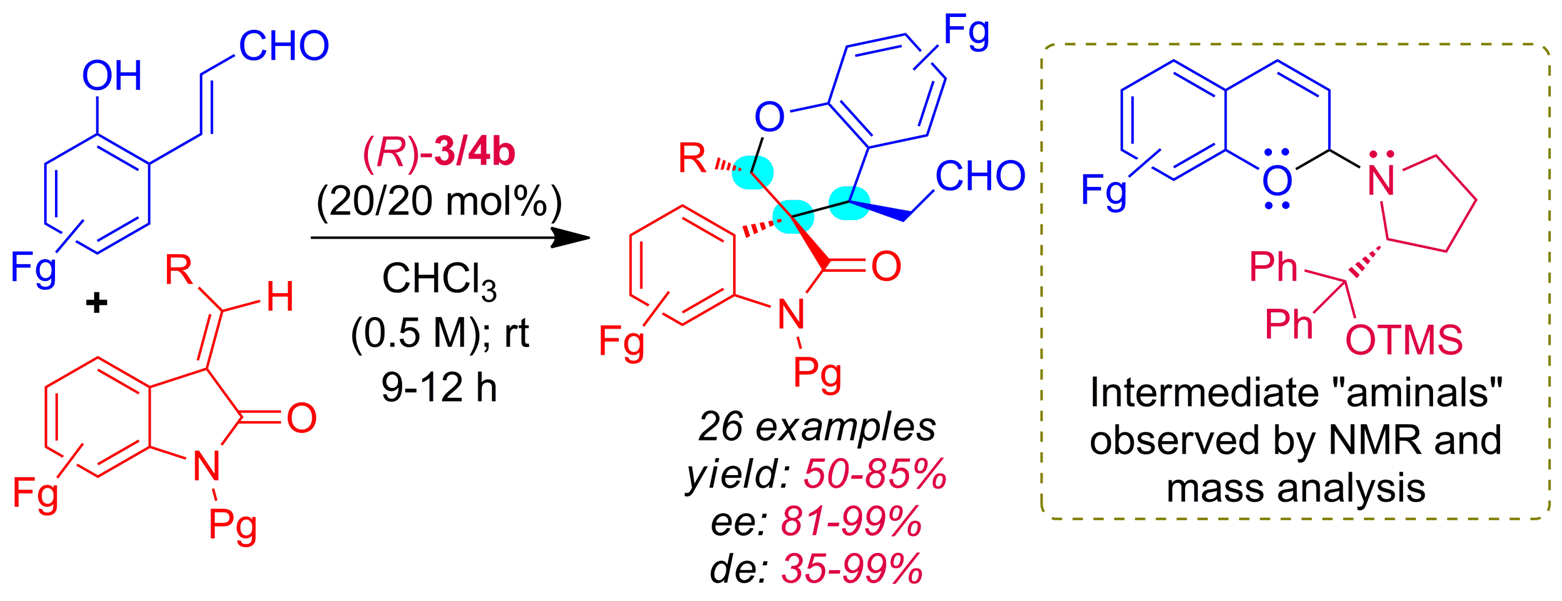
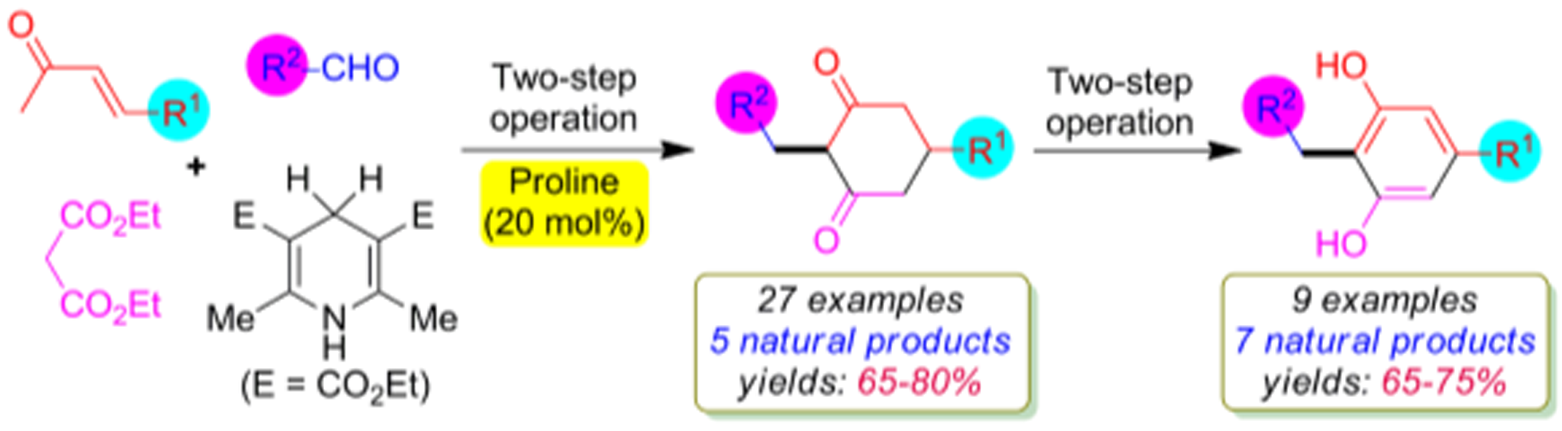
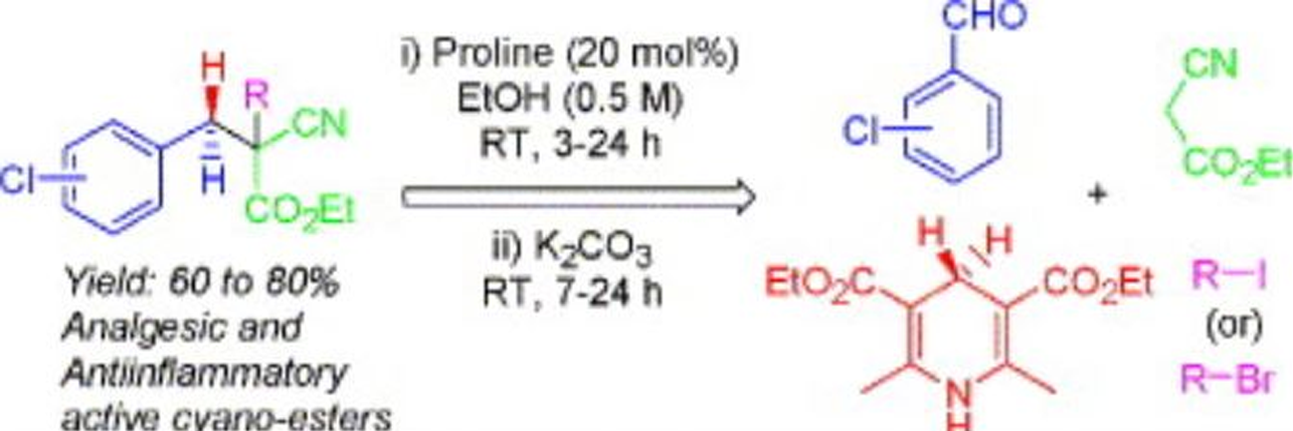
Org. Biomol. Chem.2022
Research Interests
Synthetic Organic Chemistry (Both Total Synthesis and Reaction Engineering)
Asymmetric Supramolecular Catalysis and Organocatalysis
Development of Multi-Component and Multi-Catalysis Cascade Reactions
Application of Organocatalysis in Other Disciplines
Usefull Links
Recent Publications
Tetrahedron 2022
Lawsone as Synthon in the Catalytic Asymmetric Reactions
Copyright © 2020 Guruvalar Info Tech. All rights reserved.