Publication Details:
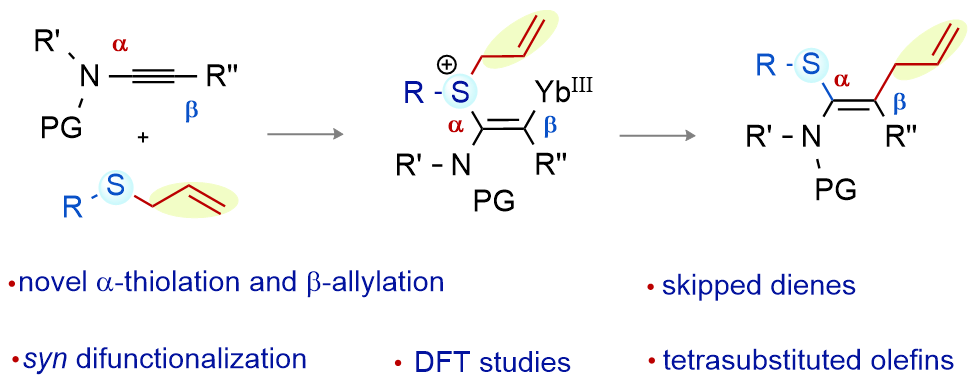
Title: Yb(III)-catalysed syn-thioallylation of ynamides
| Author(s) | Manash Protim Gogoi, Rajeshwer Vanjari, B. Prabagar, Shengwen Yang, Shubham Dutta, Rajendra K. Mallick, Vincent Gandon, Akhila K. Sahoo* : Chem. Commun., 2021, 57, 7521-7524. |
|---|---|
| Journal Name | Chemical Communications |
| journal image URL | http://chemistry.uohyd.ac.in/~aks/wp-content/uploads/bhanuccom_cover.png |
More Details:
Reported herein is a syn-thioallylation of ynamides incorporating a sulfide moiety at the α-position and an allyl group at the β-position of the ynamide. The transformation is successful under ytterbium(III)-catalysis, providing access to highly substituted thioamino-skipped-dienes with broad substrate scope. Thus, tetrasubstituted olefins (with four different functional groups: amide, phenyl, thioaryl/alkyl, and allyl on the carbon centers) are made in a single step from readily accessible ynamides, preserving complete atom economy. The reaction can be extended to the synthesis of selenoamino dienes by ynamide syn-selenoallylation. DFT studies and control experiments provide insight into the reaction mechanism.
https://pubs.rsc.org/en/content/articlehtml/2021/cc/d1cc02611a