Publication Details:
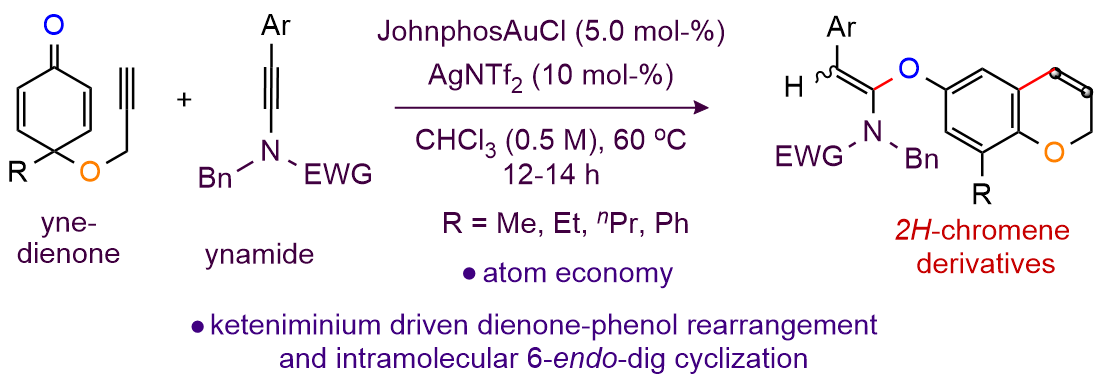
Title: Keteniminium Induced Dienone-Phenol Rearrangement and Intramolecular 6-endo-dig Cyclization Cascade of Yne-Dienone
| Author(s) | Rajendra K. Mallick, Shubham Dutta, Manash Protim Gogoi, and Akhila K. Sahoo* (invitation) |
|---|---|
| Journal Name | Helvetica Chimica Acta |
| journal image URL | http://chemistry.uohyd.ac.in/~aks/wp-content/uploads/Helvetika logo.jpg |
More Details:
Demonstrated herein is a keteniminium triggered dienone-phenol rearrangement of yne-dienone and a regioselective intramolecular 6-endo-dig cyclization of aryl-propargyl ether cascade for the synthesis of 2H-chromene bearing ketene-N,O-acetals. The gold-catalyst and ynamide combination forms a keteniminium species in-situ and makes the dienone-phenol rearrangement viable with 1,2-migration of common alkyl substituents (methyl, ethyl, and n-propyl) and phenyl group. The transformation tolerates common functional groups and N-sulfonyl protecting groups in ynamides exhibiting broad scope. The transformation proceeds through intramolecular proton transfer to the vinylic gold species; deuterium scrambling study supports this observation.