Publication Details:
Title: Keteniminium Driven Umpolung Difunctionalization of Ynamides
| Author(s) | Shubham Dutta, Shengwen Yang, Rajeshwer Vanjari, Rajendra Kumar Mallick, Vincent Gandon, and Akhila Kumar Sahoo : Angew. Chem., Int. Ed. 2020, 59, 10785–10790 |
|---|---|
| Journal Name | Angewandte Chemie Int. Ed. |
| journal image URL | http://chemistry.uohyd.ac.in/~aks/wp-content/uploads/2020 angew new.png |
More Details:
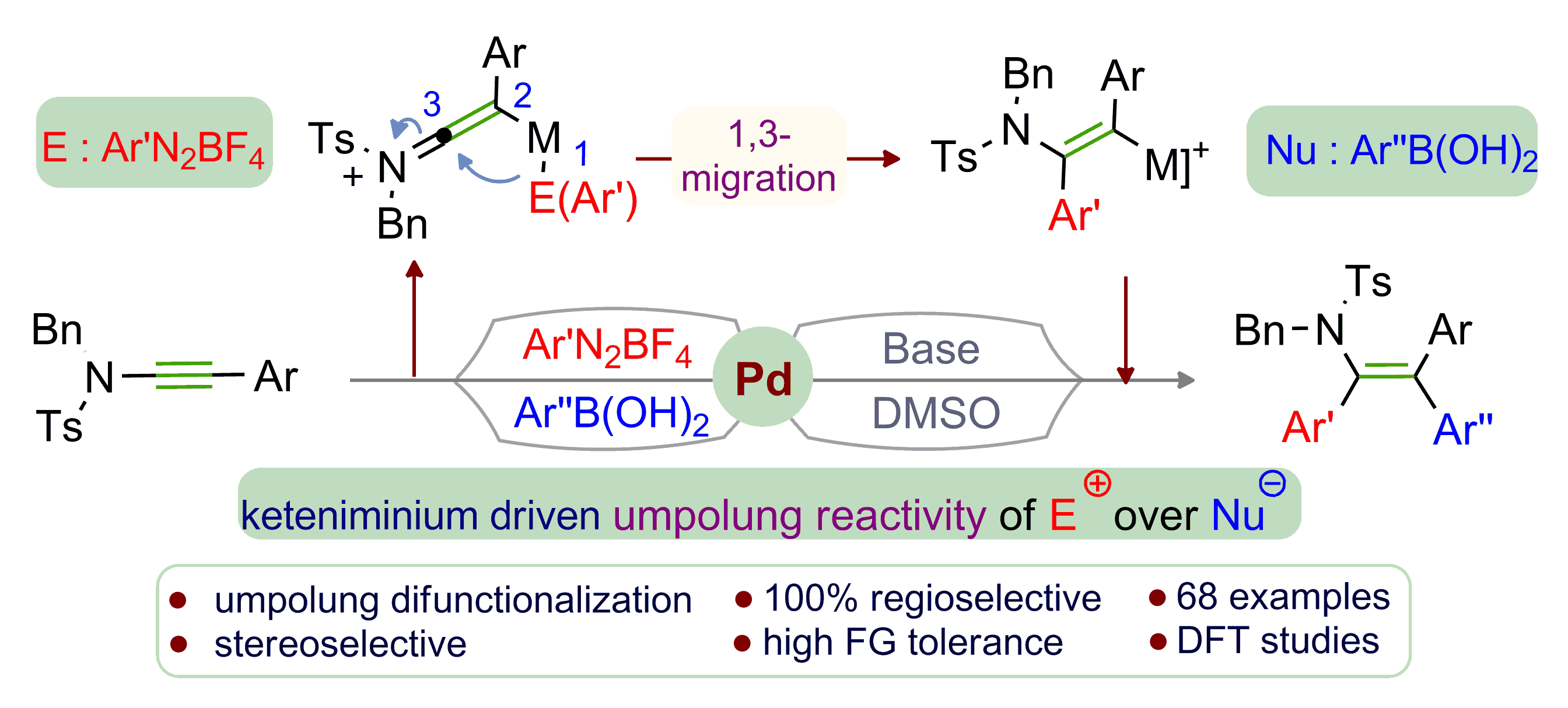
A three component Pd‐catalyzed coupling of ynamides, aryl diazonium salts, and aryl boronic acids for the synthesis of novel triaryl‐substituted enamides is described. This transformation represents the first example of an umpolung regioselective unsymmetrical syn‐1,2‐diarylation/aryl‐vinylation of ynamides. The aryl moieties of diazonium salt (electrophile) and boronic acid (nucleophile) are explicitly incorporated in the electrophilic α‐ and nucleophilic β‐position of the ynamide, respectively, resulting in a single isomer of N‐bearing tetrasubstituted olefin. The scope is broad (67 examples) showing excellent functional group tolerance. DFT calculations substantiate the rationale of the mechanistic cycle and the regioselectivity. Chemoselectivity and synthetic potential of the enamide products are also studied.