Publication Details:
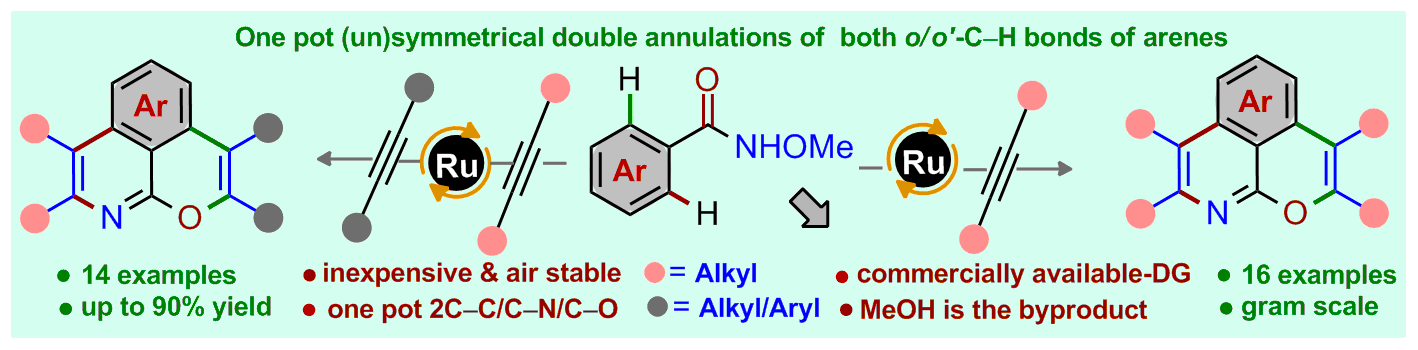
Title: Construction of Pyranoisoquinolines via Ru(II)-Catalyzed Unsymmetrical Double Annulation of N-Methoxybenzamides with Unactivated Alkynes
| Author(s) | Tirumaleswararao Guntreddi, Majji Shankar, Nagarjuna Kommu and Akhila K. Sahoo : J. Org. Chem. 2019, 84, 20, 13033-13044 |
|---|---|
| Journal Name | The Journal of Chemistry |
| journal image URL | http://chemistry.uohyd.ac.in/~aks/wp-content/uploads/joctiru.png |
More Details:
A ruthenium (Ru)-catalyzed double annulation of easily accessible N-methoxybenzamide derivatives with unactivated alkynes for the synthesis of unusual 6,6-fused pyranoisoquinolines is described. Both ortho-C-H bonds of arenes as well the N- and O-moieties of N-methoxybenzamides are involved for the construction of four [(C–C)-(C–N) and (C-C)-(C-O)] bonds in one step under single catalytic conditions. The unsymmetrical annulation of N-methoxybenzamides with two distinct alkynes is also demonstrated. The oxidizable directing group N-methoxyamine (NHOMe) assists the unsymmetrical double annulations of arenes [that uses both N- and O- heteroatoms] in a single operation. This synthetic method features excellent substrate scope and tolerates wide range of functional groups. Peripheral modification of pyranoisoquinolines for the construction of complex heterocyclic compounds is also demonstrated.