Publication Details:
Title: Metal-free stereoselective addition of propiolic acids to ynamides: a concise synthetic route to highly substituted enediyne/dienyne-(E)-N,O-acetals
| Author(s) | Rangu Prasad, Suresh Kanikarapu, Shubham Dutta, Srinivas Vangara and Akhila K. Sahoo. New J. Chem., 2022, Advance Article. |
|---|---|
| Journal Name | New Journal of Chemistry |
| journal image URL | http://chemistry.uohyd.ac.in/~aks/wp-content/uploads/NJC 2022.jpg |
More Details:
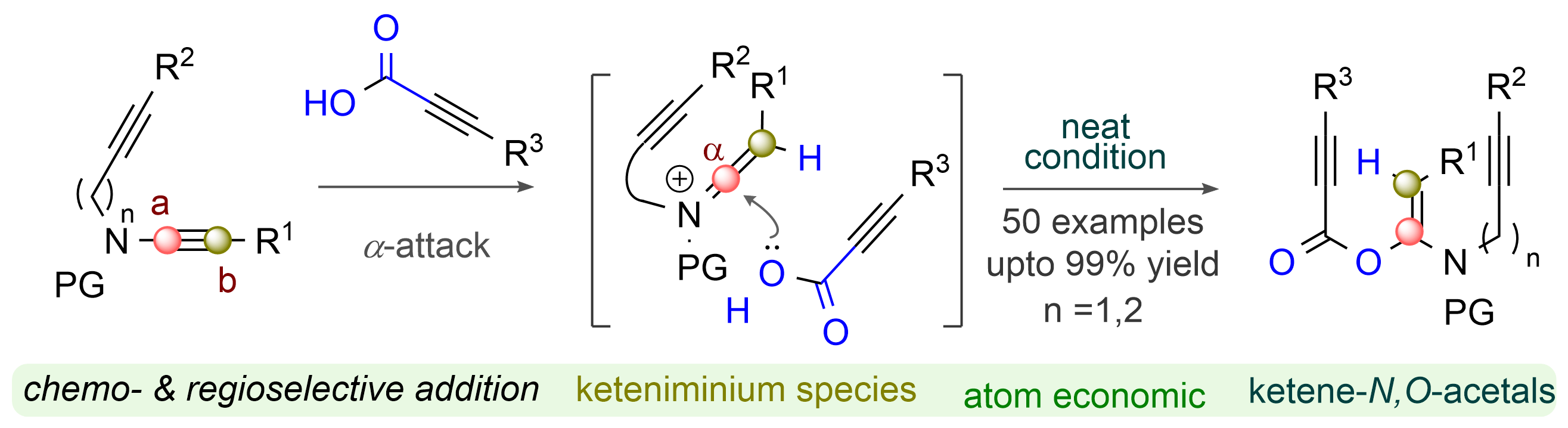
A straightforward and sustainable approach for the 1,2-addition of propiolic acids to ynamide has led to bench-stable sp2 (E)-enol-enamides of enediynes and dienynes. The reaction is chemo-, regio-, and stereoselective and does not require metal catalysts and solvent. The ynamide motif is extremely amenable to functionalization in the presence of the tethered alkyne triple bond. The synthetic technique is operationally simple; no hydrolysis of ketenimine species occurs under acidic conditions. The transformation tolerates a wide range of common functional groups, constructing a library of highly functionalized 1,5,5′/6′-enediynes and 1,5,5′-dienynes of sp2 (E)-N,O-acetal skeletons.
https://pubs.rsc.org/en/content/articlehtml/2022/nj/d2nj01907h