Publication Details:
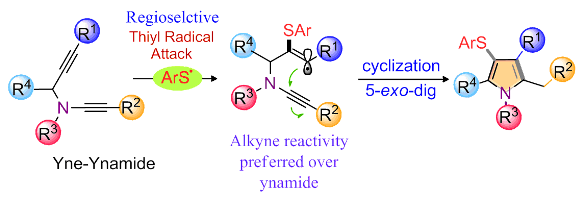
Title: Alkyne versus Ynamide Reactivity: Regioselective Radical Cyclization of Yne‐Ynamides
| Author(s) | Shubham Dutta, Rajendra K Mallick, Rangu Prasad, Vincent Gandon, and Akhila Kumar Sahoo : Angew.Chem. Int.Ed. 2019, 58,2289 –2294 |
|---|---|
| Journal Name | Angewandte Chemie International Edition |
| Link to | https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201811947 |
| journal image URL | http://chemistry.uohyd.ac.in/~aks/wp-content/uploads/prabagaranie.png |
More Details:
Ynamides are typically more reactive than simple alkynes and olefins. However, a serendipitous observation revealed a rare case where the reactivity of simple alkynes exceeds that of ynamides. This led to the development of a unique sulfur radical‐triggered cyclization of yne‐tethered‐ynamides, which involves the attack of a thiyl radical to the alkyne and then that of the ynamide. A wide range of novel 4‐thioaryl pyrroles that could tolerate common functional moieties and N‐protecting groups were expediently constructed by this strategy. The current method opposes to the typical cyclization of yne‐ynamides, which involves the attack of the ynamide core to the alkyne moiety. Control experiments and DFT calculations supported the participation of the sulfur radical in the reaction and the regioselective cyclization. The synthetic potential of the substituted pyrroles is also discussed.