Publication Details:
Title: Sulfur and Nitrogen Modulated One-Pot Double Annulation of Arenes
| Author(s) | Majji Shankar, Arijit Saha, Arghadip Ghosh, Somratan Sau, and Akhila K. Sahoo* : J. Org. Chem. 2021, 86, 14942–14955. |
|---|---|
| Journal Name | The Journal of Organic Chemistry |
| journal image URL | http://chemistry.uohyd.ac.in/~aks/wp-content/uploads/JOC sulfur logo.jpg |
More Details:
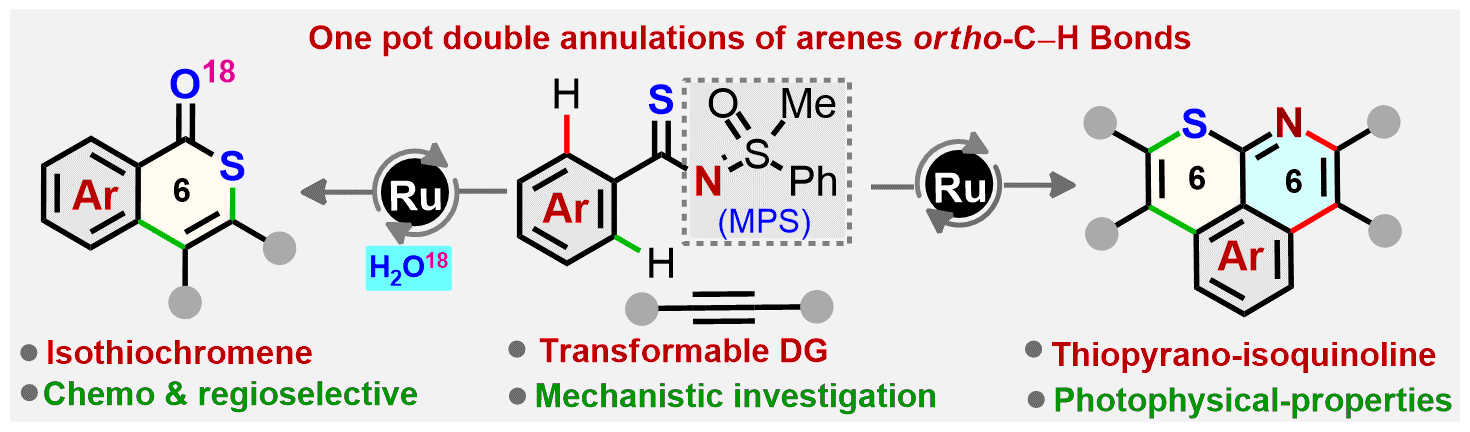
The sulfur and nitrogen moieties of methylphenyl sulfoximine (MPS)-enabled aryl thioamides are independently involved in annulation with unactivated alkynes to construct the unusual 6,6-fused thiopyranoisoquinoline skeletons. The MPS directing group plays a vital role in making this unprecedented Ru-catalyzed one-pot double annulation of aryl thioamides with alkynes chemo- and regioselective. Both the o,o′-C–H bonds of the aryl motif are sequentially functionalized to form four bonds [C–C, C–S and C–C, C–N] in a single operation by overcoming the undisputed challenges, viz. the “S” poisoning effect on the transition-metal catalyst and the susceptibility of S to oxidation. The novel isothiochromene-1-one skeletons are successfully constructed, as the annulation is initiated with S in preference over the N motif of thioamides with alkynes. The preliminary photophysical properties of the thiopyrano-isoquinoline derivatives are also discussed.