Publication Details:
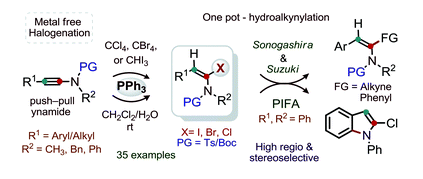
Title: Triphenylphosphine promoted regio and stereoselective α-halogenation of ynamides
| Author(s) | B Prabagar, Sanatan Nayak, Rajendra K Mallick, Rangu Prasad, Akhila K Sahoo : Organic Chemistry Frontiers, 3 (1), 110-115 |
|---|---|
| Journal Name | Organic Chemistry Frontiers |
| journal image URL | http://chemistry.uohyd.ac.in/~aks/wp-content/uploads/prabaocf_cover.png |
More Details:
In this study, we demonstrate metal free regio- and stereoselective α-halogenation (Cl, Br and I) of ynamides. The halogenation of ynamides in the presence of PPh3 and CCl4, CBr4 or CHI3 in moist CH2Cl2 at room temperature provides good-to-excellent yields of a wide variety of stable (E)-α-haloenamides; thus, the reaction has a broad scope. Chlorination of ynamides at room temperature is particularly notable. The sequential one pot α-iodination and alkynylation of ynamides provide synthetically useful amidoenynes. The chlorination of 3-acetoxy ynamide delivers 3-acetoxy-α-chloro enamide without the migratory-assistance of the acetoxy moiety.
https://pubs.rsc.org/en/Content/ArticleLanding/2016/QO/C5QO00345H