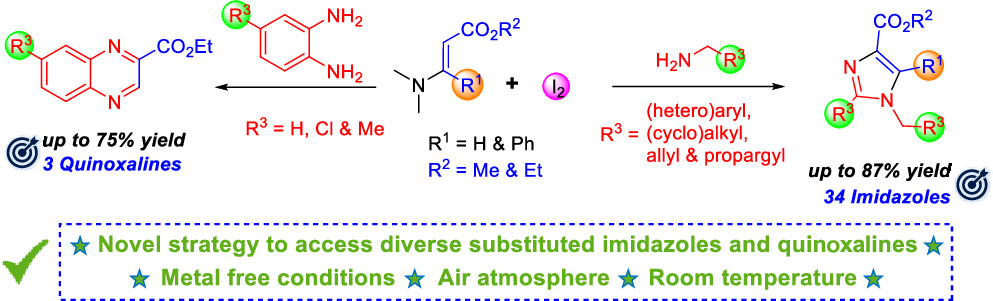
30) Synthesis of Diverse Imidazole and Quinoxaline Derivatives via Iodine-Mediated Cyclization Reactions
J. Org. Chem. 2025, ASAP
DOI
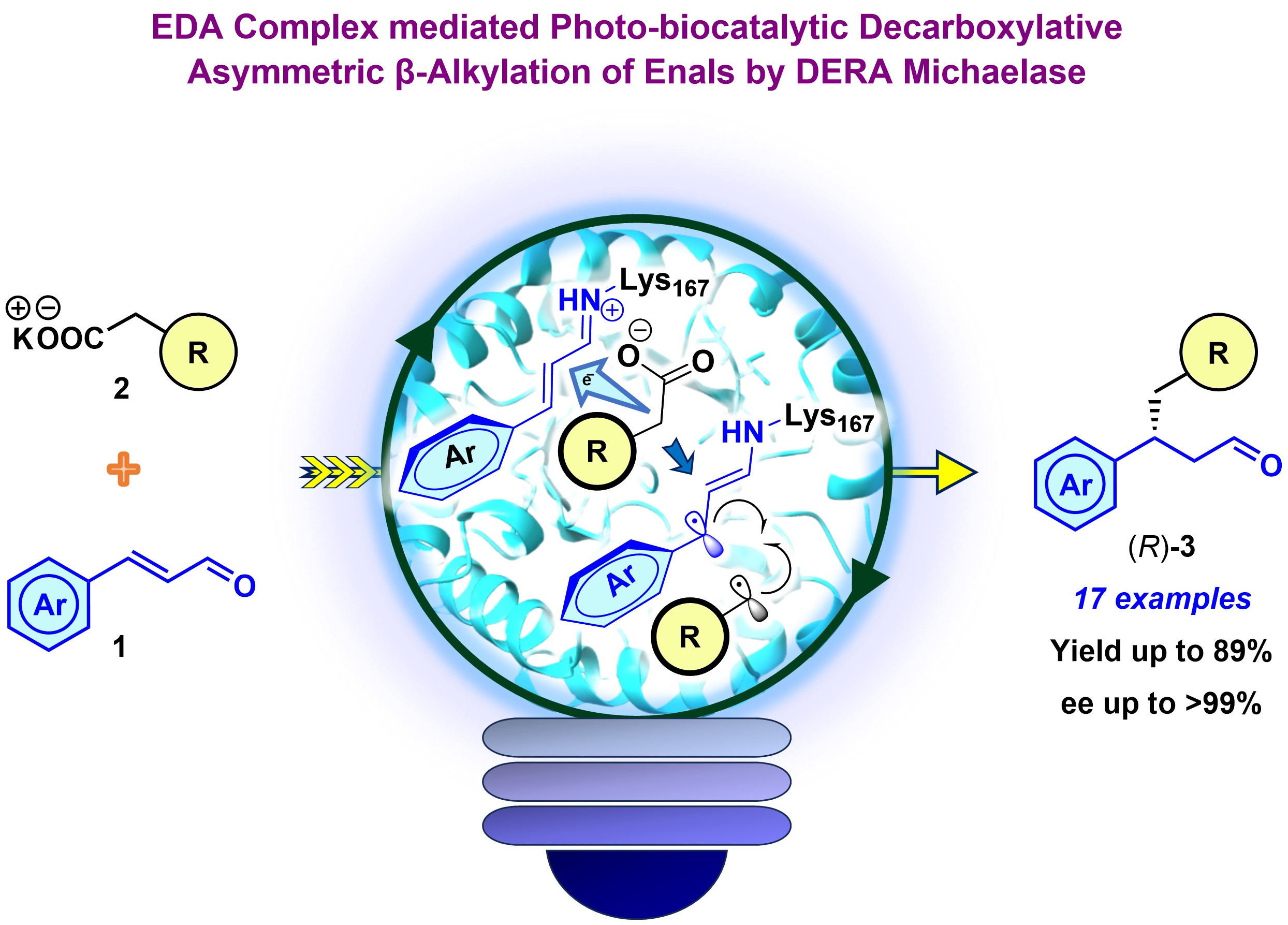
29) “Excited” Class I Aldolases: EDA Complex Mediated Photo-biocatalytic Enantioselective β-Alkylation of Enals
ACS Catal., 2025, 15, 2531 – 2539
DOI
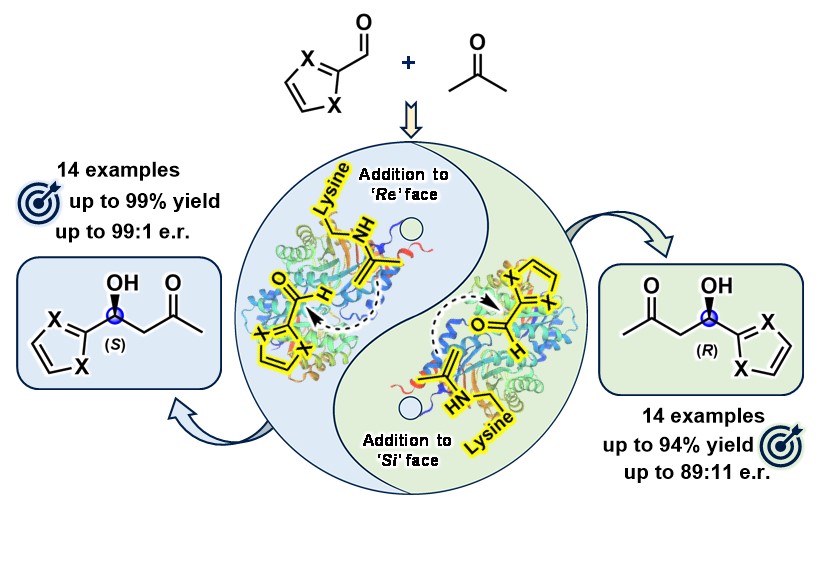
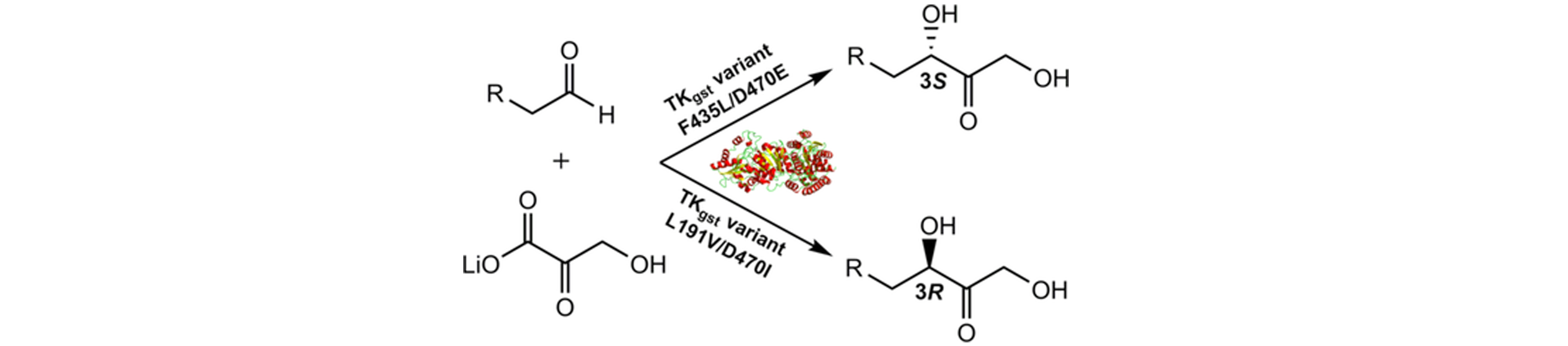
28) Enantiodivergent Synthesis of Both (R)- and (S)-Heteroaryl Aldols by Rationally Engineered Aldolases
J. Org. Chem., 2025, ASAP
DOI
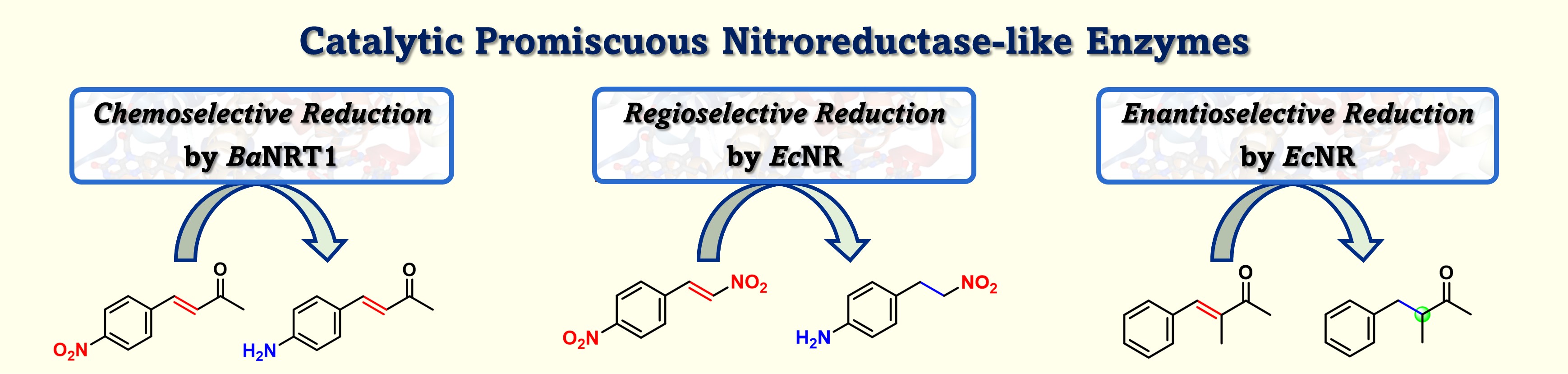
27) Exploring the Substrate Scope and Catalytic Promiscuity of Nitroreductase-Like Enzymes
Adv. Synth. Catal., 2024, 366, 4679 – 4687
DOI
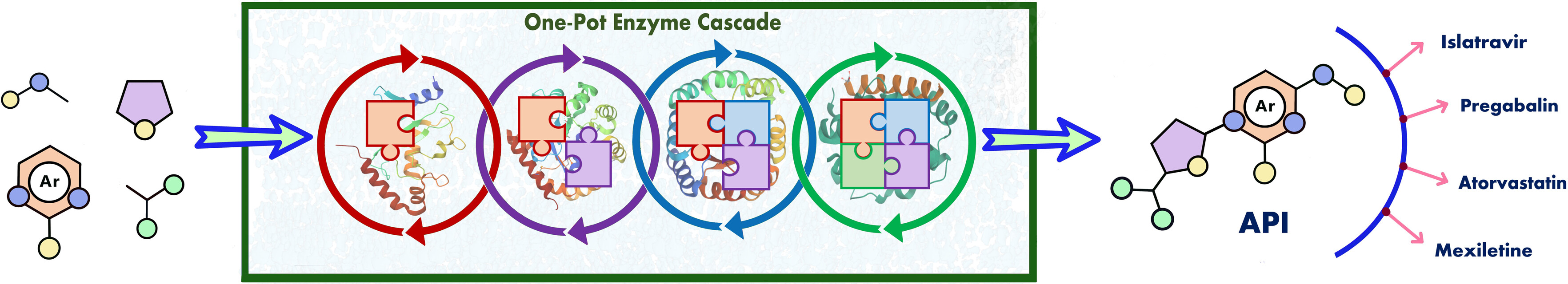
26) Evaluating Multienzyme Cascade Routes for Pharmaceutically Relevant Molecules
Eur. J. Org.Chem., 2024, e202301236
DOI
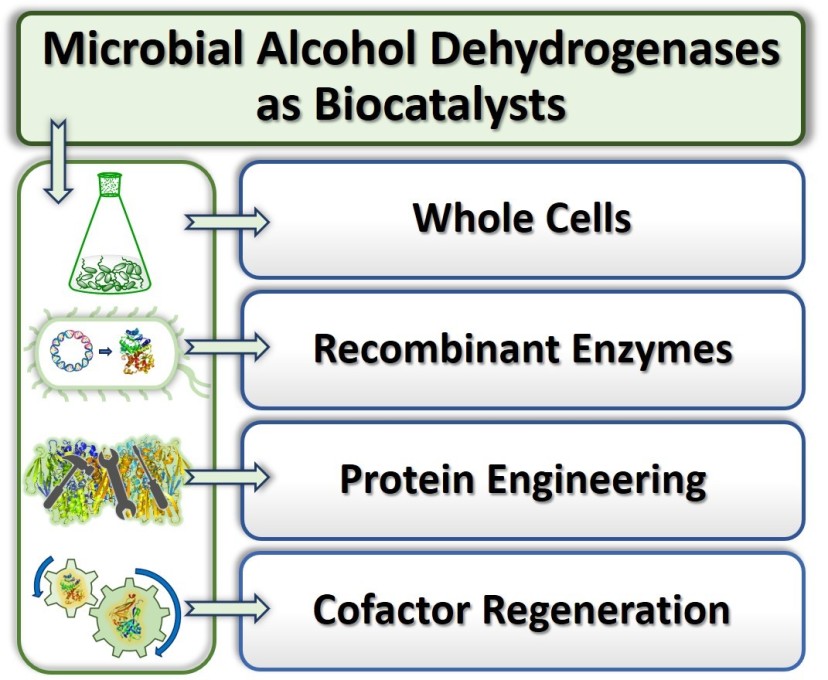
25) Microbial alcohol dehydrogenases: recent developments and applications in asymmetric synthesis
Org. Biomol. Chem., 2024,22, 228 − 251
DOI
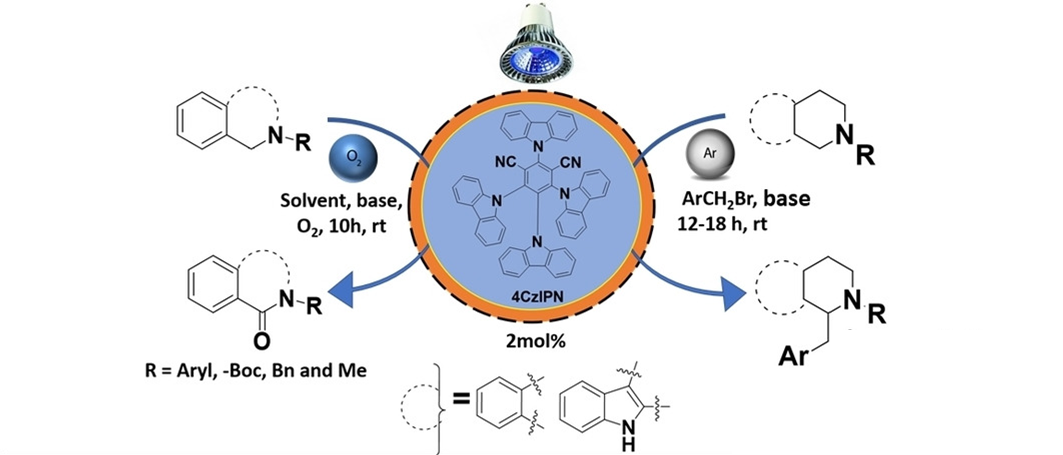
24) Visible Light Driven Metal-Free Photoredox Catalyzed α-benzylation and α-oxygenation of N-substituted Tetrahydroisoquinolines: Applications to Synthesis of Natural Products
Chem. Asian J., 2022, e202200878
DOI
23) Chemo- and Enantioselective Photoenzymatic Ketone Reductions Using a Promiscuous Flavin-dependent Nitroreductase
ChemCatChem, 2022, e202200043
DOI
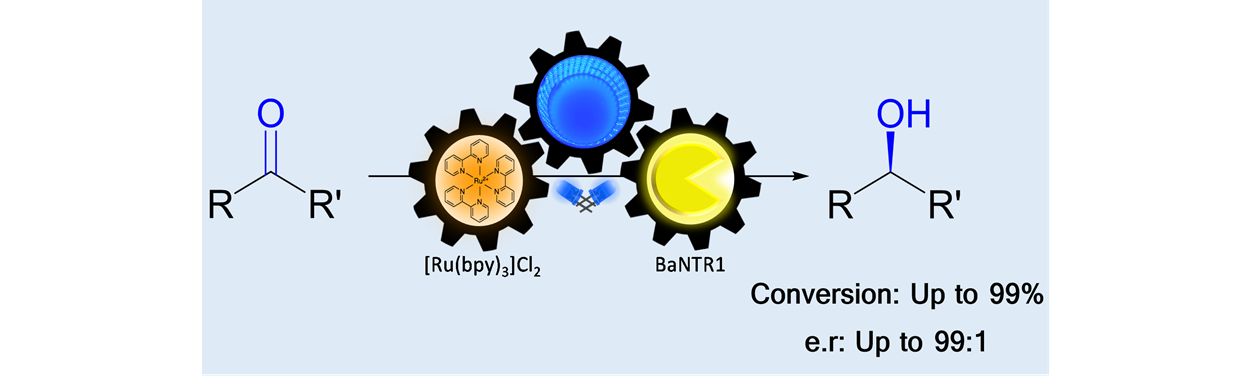
22) Biocatalytic Asymmetric Cyclopropanations via Enzyme-Bound Iminium Ion Intermediates
Angew. Chem. Int. Ed., 2021, 60, 24059 − 24063
DOI

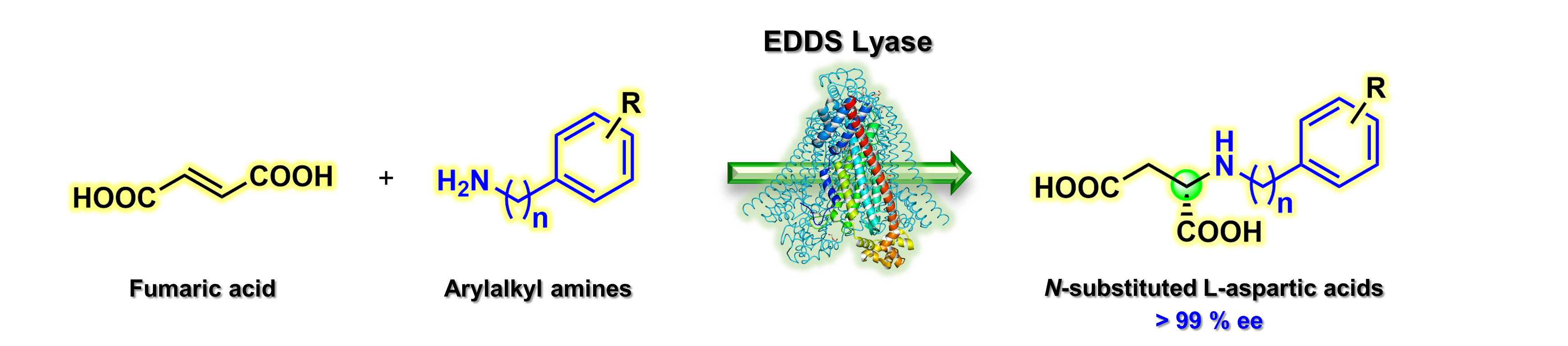
21) Biocatalytic enantioselective hydroaminations enabling synthesis of N-arylalkyl-substituted L-aspartic acids
Org. Biomol. Chem., 2021, 19, 6407 – 6411
DOI
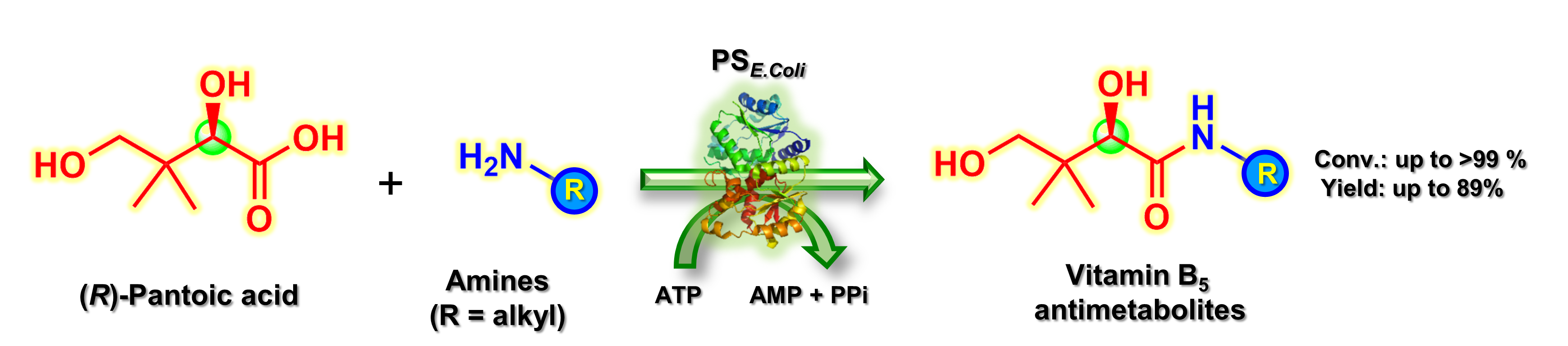
20) The broad amine scope of pantothenate synthetase enables the synthesis of pharmaceutically relevant amides
Org. Biomol. Chem., 2021, 19, 4515 – 4519
DOI
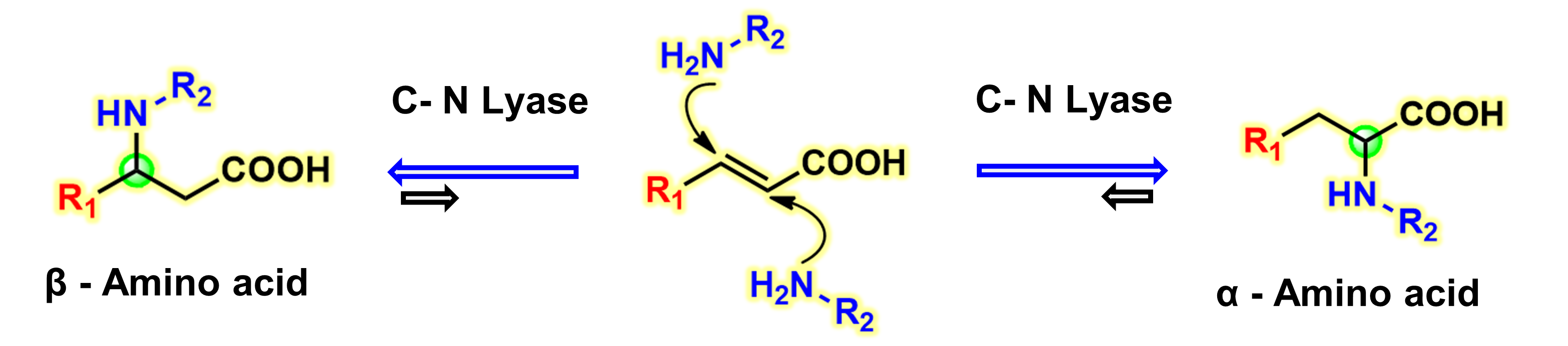
19) Recent Applications of Carbon‐Nitrogen Lyases in Asymmetric Synthesis of Noncanonical Amino Acids and Heterocyclic Compounds
ChemBioChem, 2020, 21, 2733 − 2742
DOI
18) Enantiocomplementary Epoxidation Reactions Catalyzed by an Engineered Cofactor-Independent Non-natural Peroxygenase
Angew. Chem. Int. Ed., 2020, 59, 10374 – 10378.
DOI
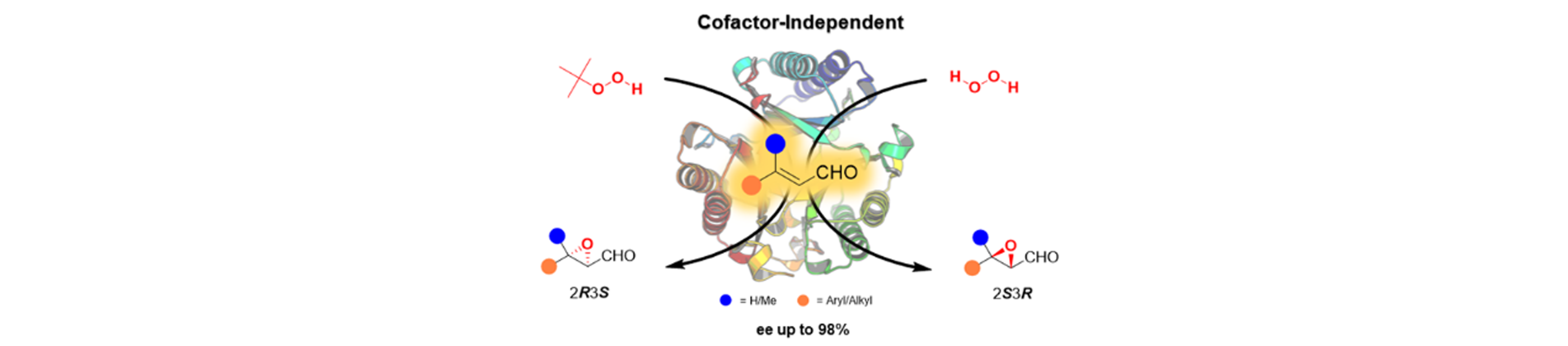
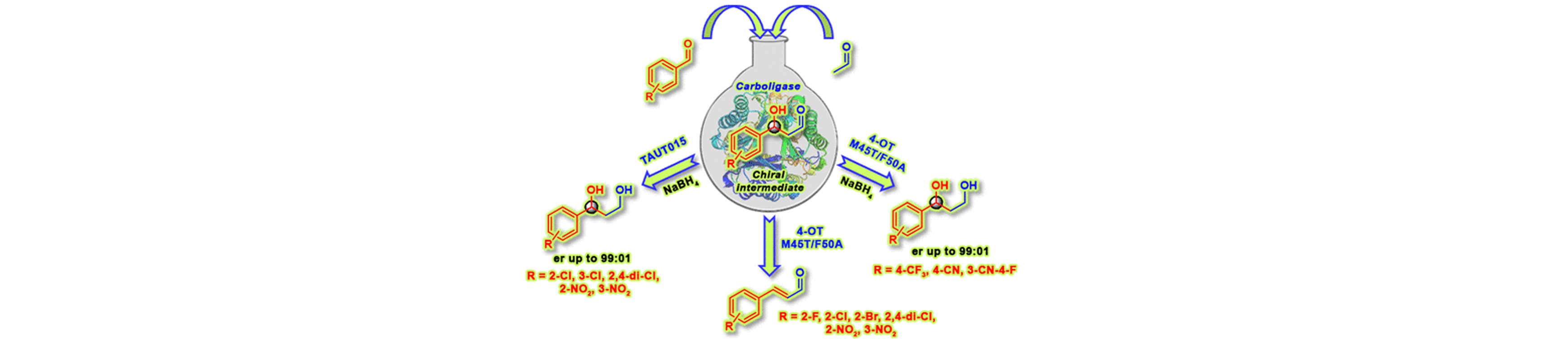
17) Enantioselective Aldol Addition of Acetaldehyde to Aromatic Aldehydes Catalyzed by Proline-Based Carboligases
ACS Catal., 2020, 10, 2522 − 2527.
DOI
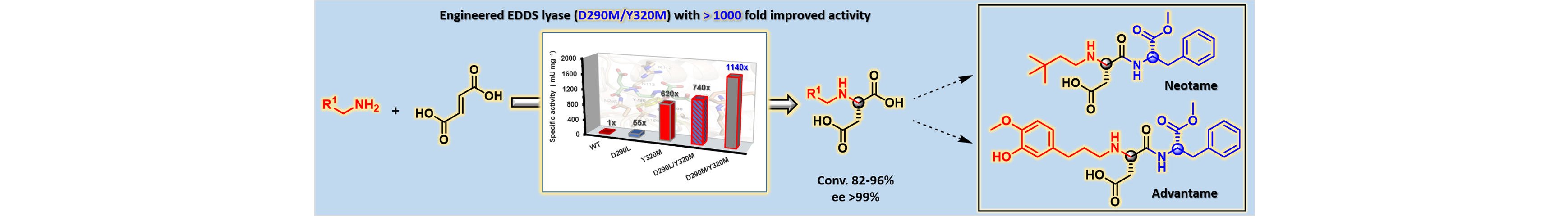
16) Engineered C–N Lyase: Enantioselective Synthesis of Chiral Synthons for Artificial Dipeptide Sweeteners
Angew. Chem. Int. Ed., 2019, 59, 429 – 435.
DOI
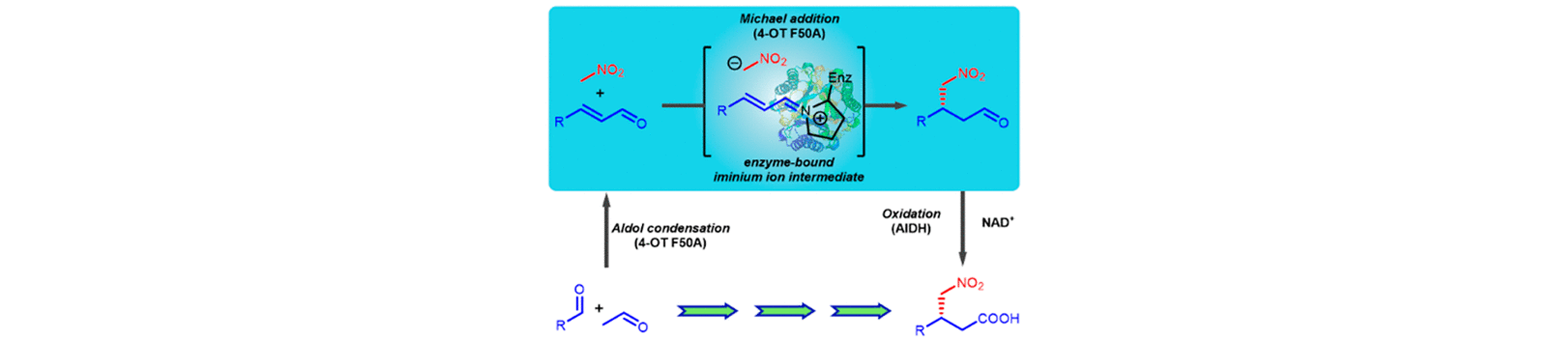
15) Biocatalytic Asymmetric Michael Additions of Nitromethane to α,β-Unsaturated Aldehydes via Enzyme-bound Iminium Ion Intermediates
ACS Catal., 2019, 9, 4369 − 4373.
DOI
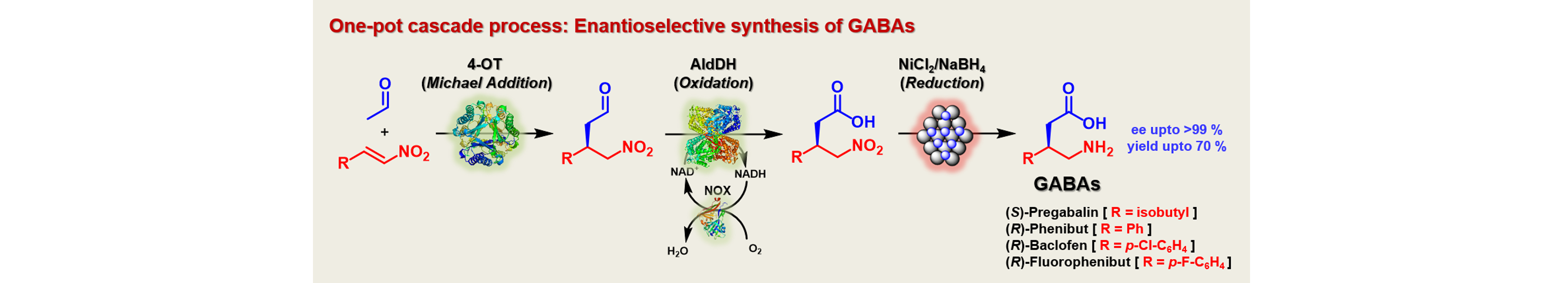
14) Enantioselective Synthesis of Pharmaceutically Active γ-Aminobutyric Acids Using a Tailor-Made Artificial Michaelase in One-Pot Cascade Reactions
ACS Catal., 2019, 9, 1503 − 1513. [ †These authors contributed equally ]
DOI
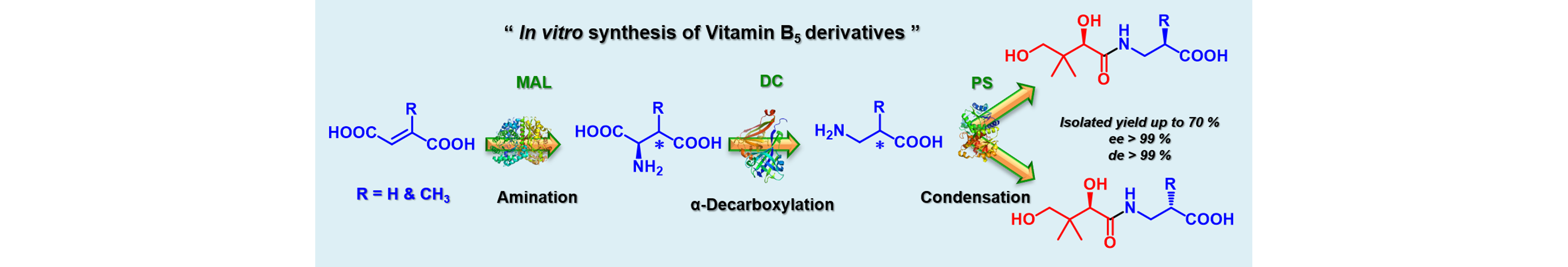
13) Modular Enzymatic Cascade Synthesis of Vitamin B5 and Its Derivatives
Chem. Eur. J., 2018, 24, 17434 – 17438.
DOI
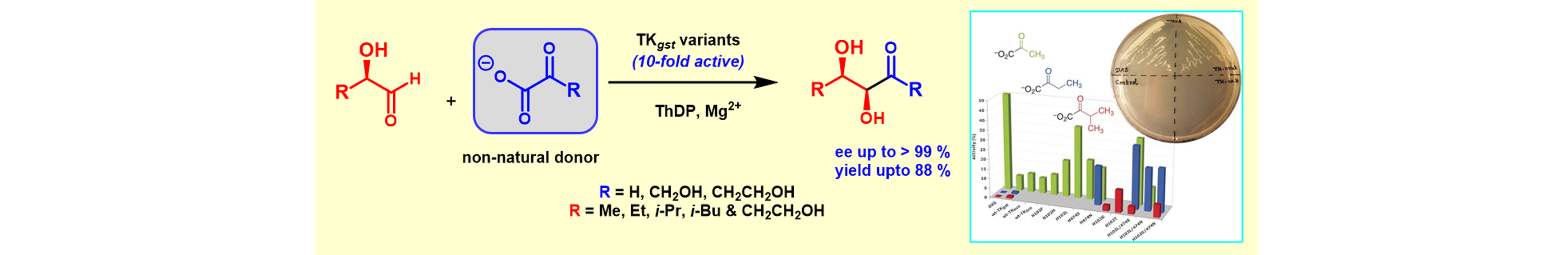
12) Donor Promiscuity of a Thermostable Transketolase by Directed Evolution: Efficient Complementation of 1-Deoxy-D-xylulose-5-phosphate Synthase Activity
Angew. Chem. Int. Ed., 2017, 56, 5358 – 5362.
DOI
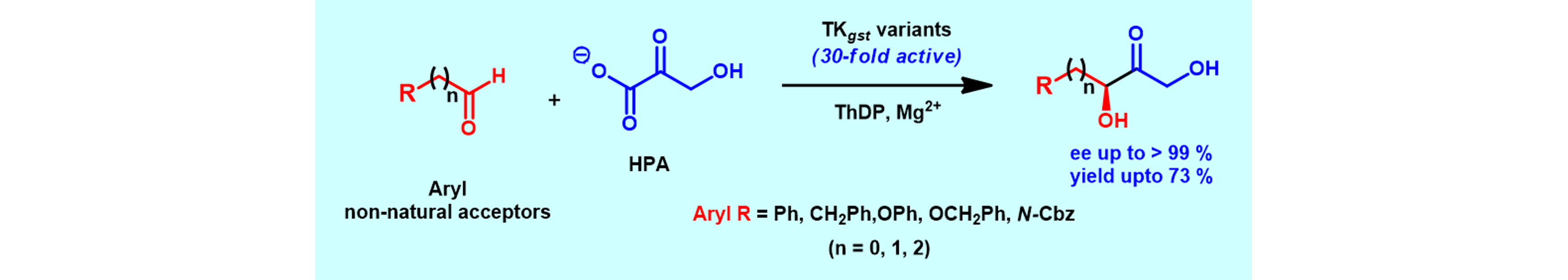
11) Engineering a Thermostable Transketolase for Arylated Substrates
Green Chem., 2017, 19, 481 – 489.
DOI
10) Second-Generation Engineering of a Thermostable Transketolase (TKGst) for Aliphatic Aldehyde Acceptors with either Improved or Reversed Stereoselectivity
ChemBioChem, 2017, 18, 455 – 459.
DOI
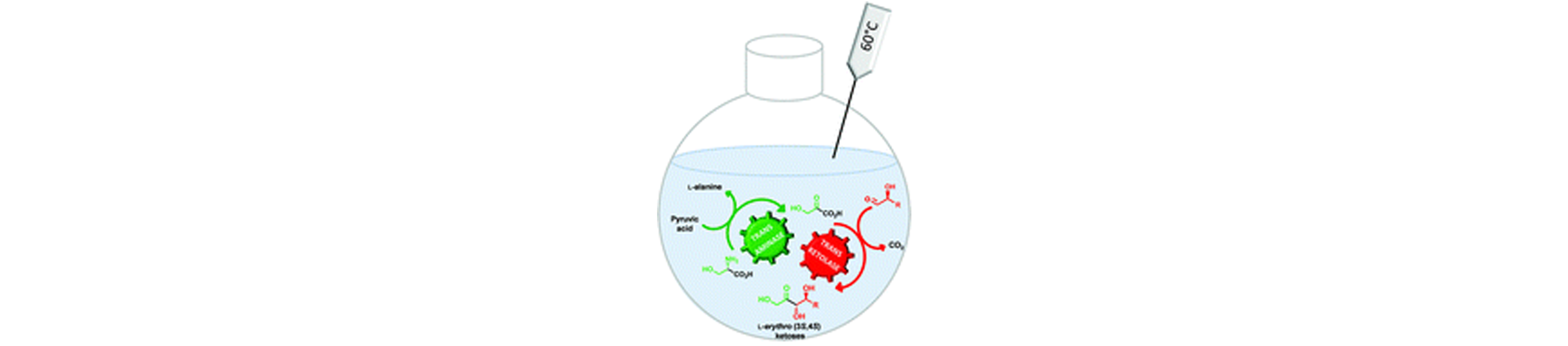
9) One-Pot, Two-Step Cascade Synthesis of Naturally Rare L-erythro (3S,4S) ketoses by Coupling a Thermostable Transaminase and Transketolase
Green Chem., 2017, 19,425 – 435.
DOI
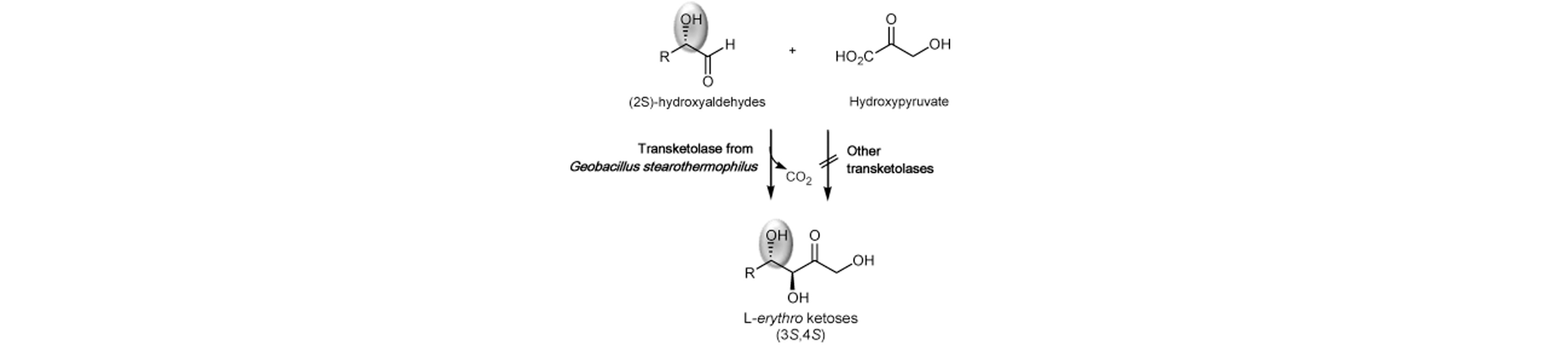
8) Engineering a Thermostable Transketolase for Unnatural Conversion of (2S)-Hydroxyaldehydes
Adv. Syn. Cat., 2015, 357, 1715 – 1720.
DOI
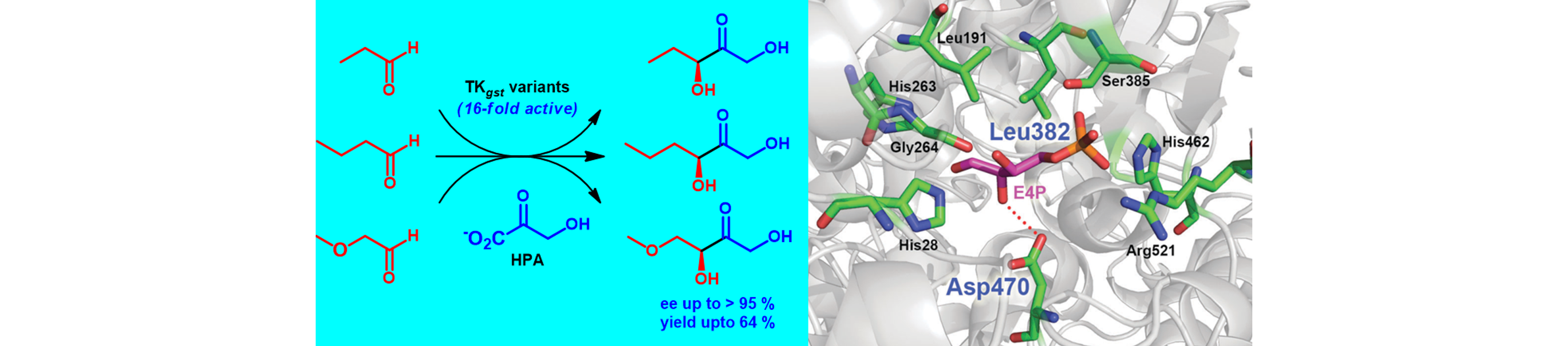
7) A Thermostable Transketolase Evolved for Aliphatic Aldehyde Acceptors
Chem. Commun., 2015, 51, 480 – 483.
DOI
6) Chemoenzymatic Synthesis of an Enantiomerically Enriched Bicyclic Carbocycle Using Candida parapsilosis ATCC 7330 Mediated Enantioselective Hydrolysis
Int. J. Org. Chem., 2015, 5, 271 – 281.
DOI
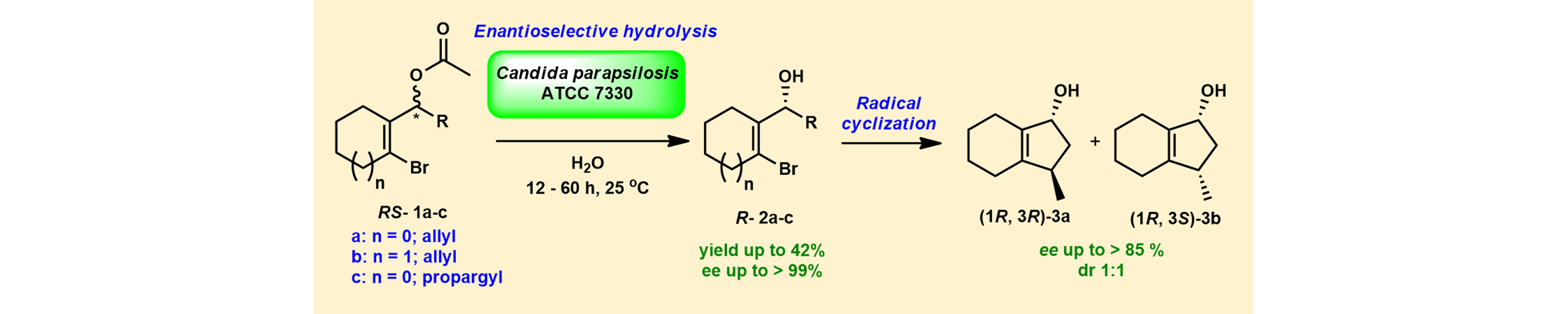
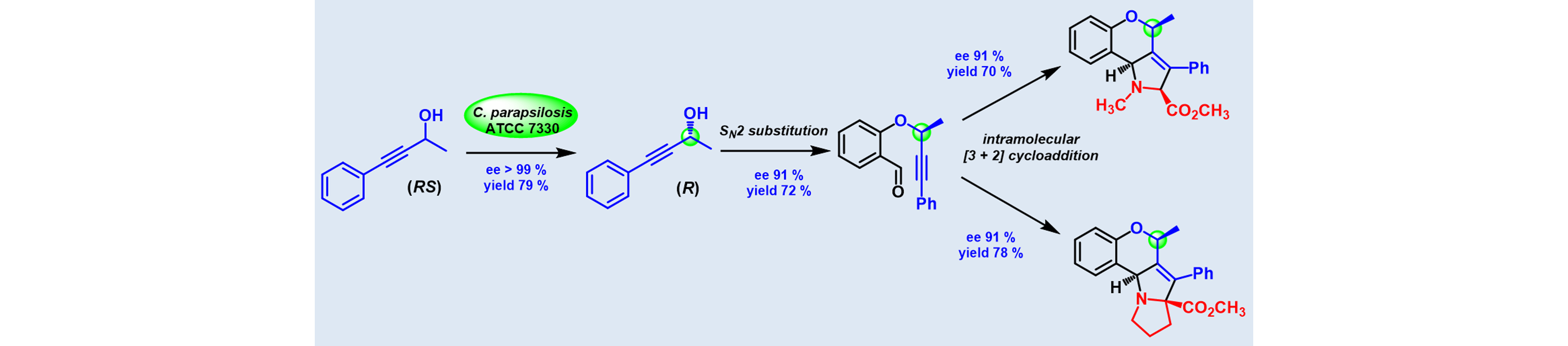
5) Utilization of Whole cell Mediated Deracemization in a Chemoenzymatic Synthesis of Enantiomerically Enriched Polycyclic Chromeno[4,3-b] pyrrolidines
Org. Biomol. Chem., 2014, 12, 4682 – 4690.
DOI
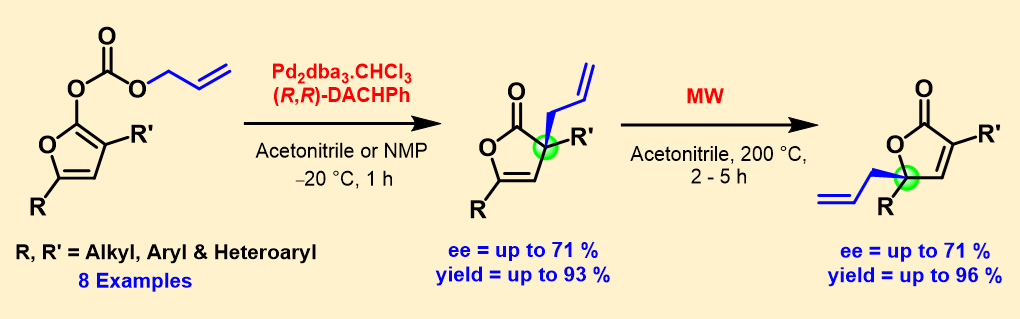
4) Palladium-Catalyzed Allylic Alkylation of Allyl Dienol Carbonates: Reactivity, Regioselectivity, Enantioselectivity and Synthetic Applications
Synlett, 2013, 24, 2350 – 2364.
DOI
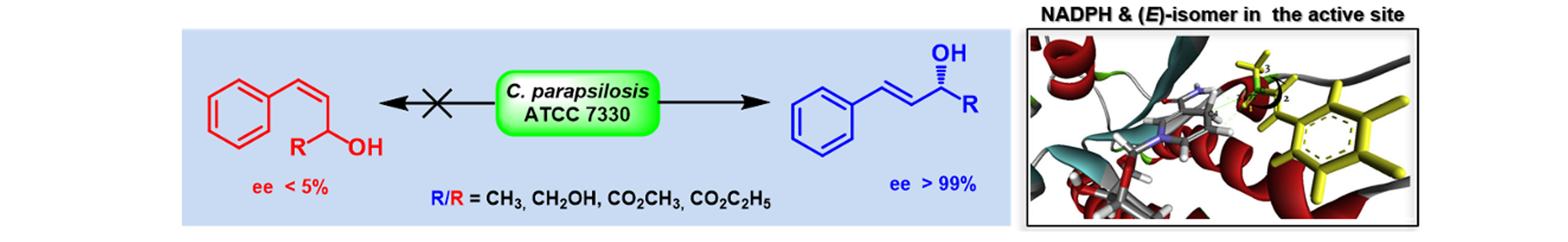
3) Stereochemical Preference in Candida parapsilosis ATCC 7330 mediated Deracemization: (E) vs. (Z)-Alkyl-2-hydroxy-4-arylbut-3-enoate
Tetrahedron: Asymmetry, 2012, 23, 1360 – 1368.
DOI
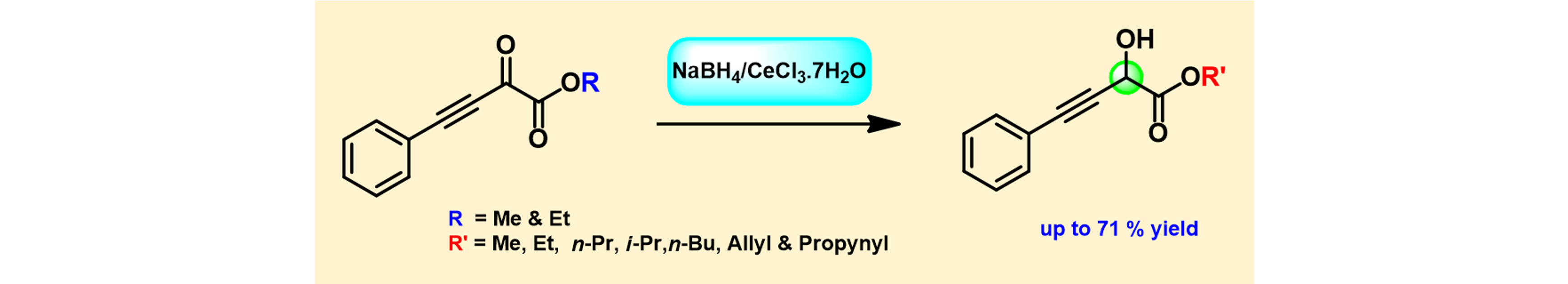
2) Chemoselective Reduction and Transesterification of α-Keto Propargylic Esters mediated by NaBH4/CeCl3.7H2O.
Syn. Comm., 2011, 41, 2350 – 2358.
DOI
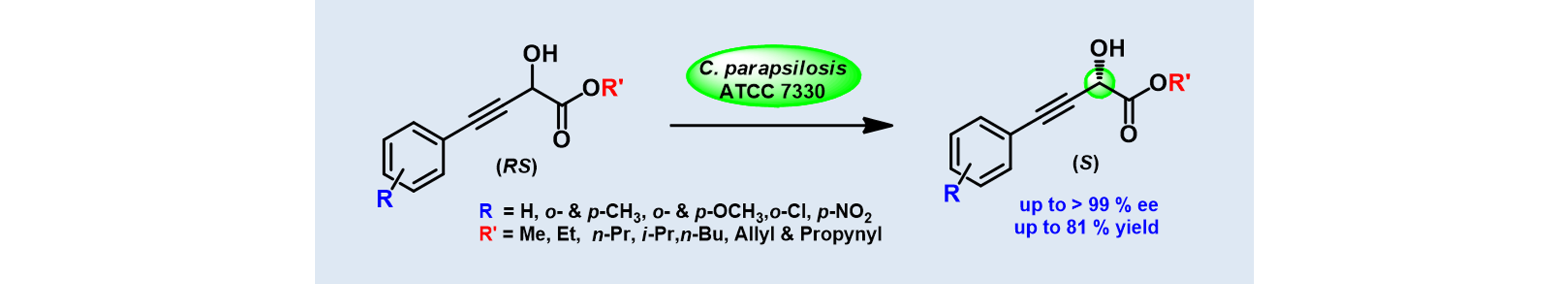
1) Biocatalytic Deracemization of Alkyl-2-hydroxy-4-arylbut-3-ynoates using Whole cells of Candida parapsilosis ATCC 7330
Tetrahedron: Asymmetry, 2010, 21, 2973 – 2980.
DOI