Research Interests & Highlights
Current Research Interests
- Organophosphorus Chemistry
- Allene chemistry
- Catalytic Organic Transformations
Selected Major Contributions from Earlier Work
-
Pentacoordinate Phosphorus Chemistry: Major insights showing the nonapplicability of commonly held tenets,
often taught and discussed in popular textbooks, has been given (Figure 1) [Representative publications:
(a) J. Am. Chem. Soc. 1996, 118, 9841;
(b) J. Am. Chem. Soc. (commun.) 2000, 122, 964-965;
(c) Acc. Chem. Res. 2006, 39, 324-333].
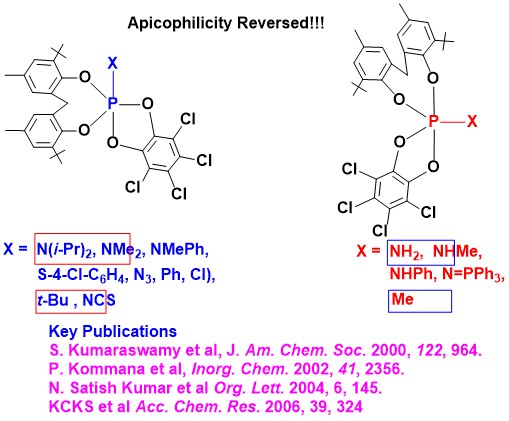
Figure 1
- Experimental Proof for Possible Phosphorus Intermediates in the Mitsunobu Reaction:
Earlier workers reported only speculative intermediates. In our work, we characterized several compounds that
were expected to be close to the proposed intermediates (Figure 2). This work culminated in a major review [Chem. Rev.
2009, 109, 2551].
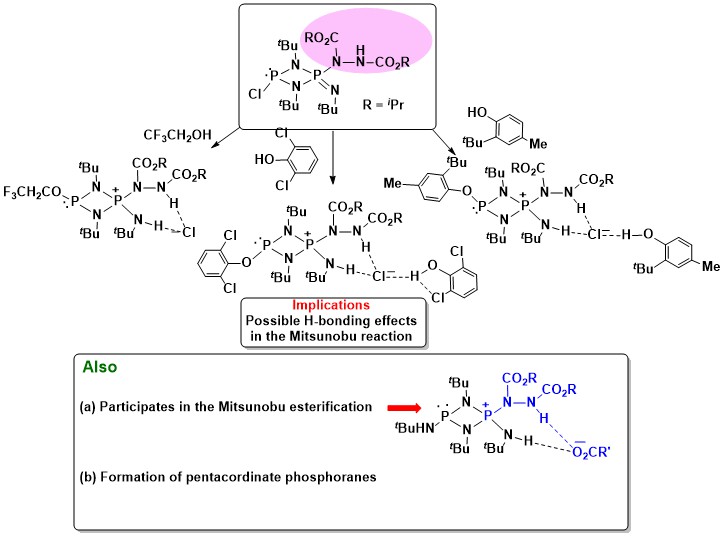
Figure 2
- H-bonding in Phosphates with Possible Implications in Biology:
Here a major contribution to our understanding of the role of H-bonding in phosphates has been made although
this part has not been pursued later (Figure 3) [J. Am. Chem. Soc. 2001, 123, 12642].
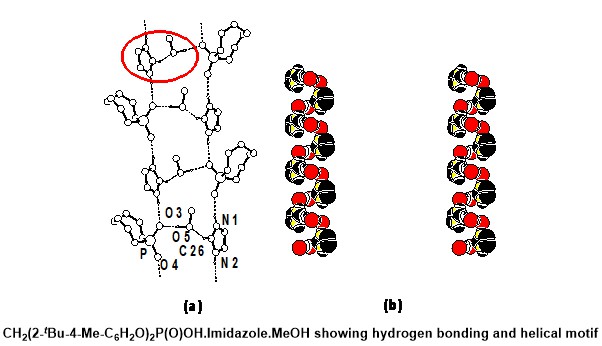
Figure 3
- Stereoselective Hydrogenation of Ynamides: Use of ethanol as a green hydrogenating agent in
ynamide chemistry has been discovered (Figure 4) [Angew. Chem. Int. Ed. 2017, 56, 6984].
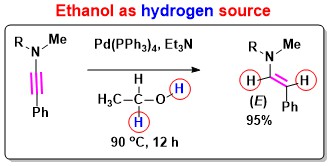
Figure 4
- Summary of Achievements
We have made contributions to the chemistry of allenoates/allenes and ynamides as well as propargylic systems. Our current work involves the utility of acetoxy allenoates and epoxy tethered substrates including ynamides. Because of the two cumulative double bonds and possible distinct substitutions at the terminal sp2 hybridized carbon atoms, allenes exhibit versatile and regioselective reactivity. These have been explored extensively in our laboratory.
- Major Contributions
- Two distinct pathways involving tertiary amine-controlled chemo-, regio-, and stereo-selective carbo-annulation
between δ-acetoxy allenoates and isothiazole dioxides with the allenoate acting as a 4C-synthon have been
demonstrated. Thus DABCO prompted diastereoselective spiroannulation offering multiple chiral centered spirocyclic
scaffolds from allenoates has been discovered. By contrast, DMAP catalyzed benzannulation using allenoates leads to
m-teraryls that are substitutionally different from that using phosphine catalysis (Scheme 1).
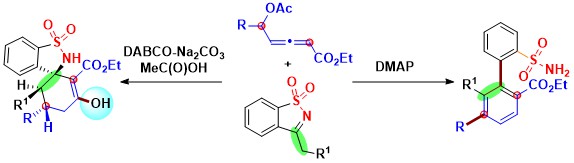
Scheme 1
- An efficient [Pd]-catalyzed synthesis of diverse sultams utilizing ynamides and boronic acids is disclosed.
This strategy has been elegantly applied to obtain dihydrobenzo[d]isothiazole-1,1-dioxides and dihydro-2H-benzo[e]
[1,2]thiazine-1,1-dioxides (Scheme 2). This protocol offers good functional group tolerance, broad substrate scope,
high-yield and low catalyst loading to access benzofused sultams possessing five- or six-membered rings.
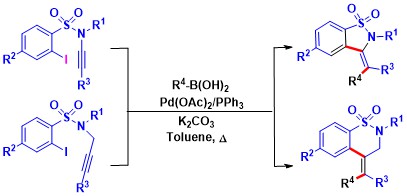
Scheme 2
- The reactivity of acetoxy allenoates with 2-sulfonamidoindoles under phosphine catalysis depends on the
disposition of the -OAc group on the allenoate. In the temperature-regulated [3 + 3] annulations, δ-acetoxy
allenoates afford dihydro-carboline and carboline scaffolds with 2-sulfonamidoindoles, in which allenoate functions
as β, γ and δ-carbon donor. At rt (25 oC), dihydro-α-carboline motifs are obtained through Michael addition,
1,4-proton shift, isomerization, phosphine elimination and aza-Michael addition. At a higher temperature (80 oC),
reaction using Ph3P-Cs2CO3 combination involves addition-elimination, aza-Claisen rearrangement, tosyl migration and
aromatization to give α-carbolines containing tosyl functionality. In contrast, with β′-acetoxy allenoate,
2-sulfonamidoindole acts as a carbo-nucleophile in (p-tolyl)3 P directed [4 + 1] spiro-annulation, affording
five-membered spiro-carbocyclic motifs (Scheme 3).
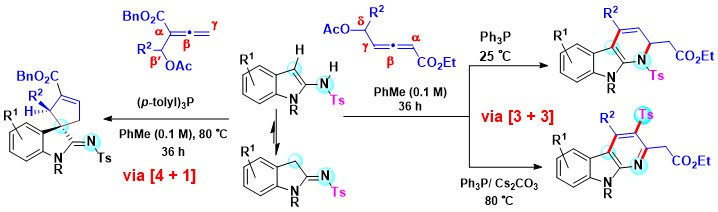
Scheme 3
- AlCl3 acts as a chlorinating agent for ynamides in the presence of stoichiometric amount of water
in dimethylcarbonate, affording (E)-α-chloroenamides. The role of water was proven by deuterium labelling
experiment. Epoxy-ynamides undergo iodochlorination and cleavage of the epoxy ring to afford (E/Z)-α-chloro-β-iodo-enamides
(Scheme 4).
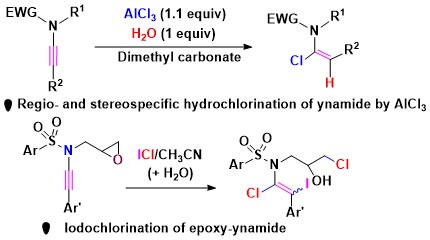
Scheme 4
- Using palladium catalysis, indole fused α-carbolines (pyridodiindoles) have been generated from 2,3′-biindoles
and nitriles. This conversion comprises double C-H activation and cyclization of 2,3′-biindoles with nitriles via
C-C and C-N bond formation. However, C-3 cyanated biindoles are formed when the reaction was performed with ethyl cyanoacetate
(Scheme 5).
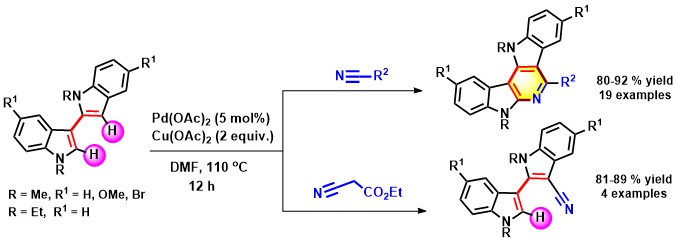
Scheme 5
- The δ-acetoxy allenoates and thioamides, under DABCO, pyridine, or tetra-n-butyl ammonium bromide
(TBAB) catalysis, undergo entirely different annulations affording chemoselective routes to dihydrothiophene, thiopyran,
or thiazole scaffolds. Thus, using pyridine, dihydrothiophenes are obtained by [3 + 2] annulation as single diastereomers.
By contrast, under DABCO catalysis, allenoates provide thiopyran motifs via 6-exo-dig cyclization. In the thiazole forming (3 + 2)
annulation, n,-Bu4NBr (TBAB) facilitates addition-elimination and 5-exo-trig cyclization in which β-
and γ-carbons of allenoates participate to afford thiazole cores with a Z-isomeric exocyclic double bond (Scheme 6).
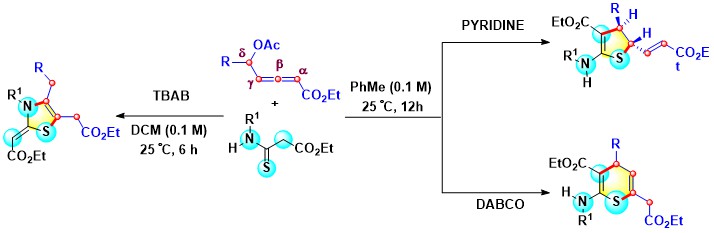
Scheme 6
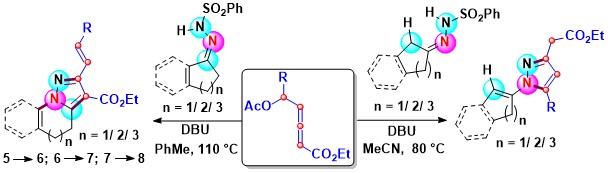
- A regio-and stereo-selective thermal [3 + 2] cycloaddition protocol involving acetoxy allenoate under metal-free conditions
for the synthesis of 1,4,5-tri/ 1,5-di- substituted-1,2,3-triazoles has been developed. The δ-acetoxy allenoate acted as an α,β-carbon
donor. By contrast, β and γ-carbons of β′-acetoxy allenoates reacted to afford 1,5-disubstituted triazole cores. This [3 + 2]
cycloaddition gives essentially E-stereoisomers (Scheme 7).
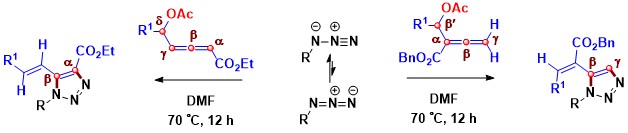
Scheme 7
- A dearomative ring expansion/spirocyclization of indole-2-carboxylates with propargylic alcohols in the presence of PTSA
leading to spiro[benzo[b]oxazine-furans via oxygen insertion is discovered; the same reactants at higher temperatures
afford fused pyrano-indolones. However, Cu(II) catalyzed annulation of indole-2-carboxylic acids with propargylic alcohols at
room temperature afforded a different class of pentacyclic indene fused pyrano-indolones (Scheme 8).
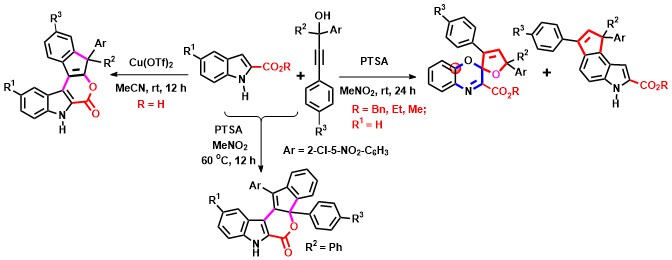
Scheme 8
- Phosphorus-containing naphthalenes have been synthesized from self-dimerization-cum-cyclization of α-aryl allenylphosphonates
or allenylphosphine oxides using the catalytic system Pd(OAc)2/PPh3/Et3N. Interestingly,
upon treating allenylphosphine oxides with Pd(OAc) 2 [stoichiometry 2:1] in the presence of PPh3/Ag2
CO3, [Pd]-complexes are isolated and structurally characterized. These complexes can be used as catalysts for C-C
bond-forming reactions of allenes with 2-iodophenol. Densely substituted 3,6-diphenylpyridazines are conveniently obtained via
thermally induced regioselective Inverse Electron Demand Diels-Alder reaction of allenes with 3,6-diphenyltetrazine (Scheme 9).
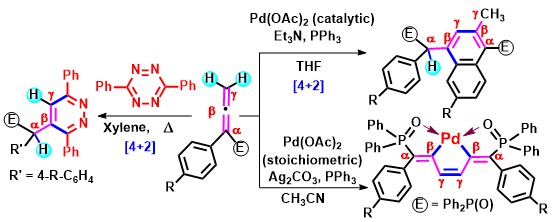
Scheme 9
- Substituted vinyl sulfonyl fluorides and ethyl diazoacetate or azides undergo (3 + 2) cycloaddition to lead to pyrazole or
triazole cores via Michael addition and SO2 elimination. Trimethylsilyl azide or organic azide selectively attacks at the β-carbon
of vinyl sulfonyl fluoride to give C-substituted or C,N-disubstituted triazoles. Vinyl sulfonyl fluorides react with ethyl diazoacetate
to form pyrazoles in good to high yields (Scheme 10).
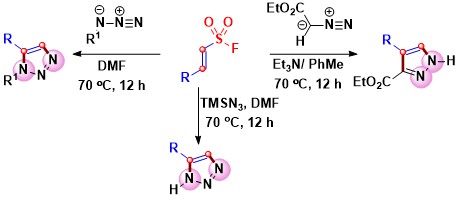
Scheme 10
- Azetidines, 2,3-dihydrofurans, and the uncommon dioxa-azabicyclononene motifs are
obtained from transition metal-free reaction between N-oxiranylmethyl
benzenesulfonamide and β-chloro-cinnamaldehyde, depending on the use of
NaI/K2CO3 or LiBr/K2CO3.
The N-oxiranylmethyl benzenesulfonamide itself upon heating gives crystalline
isomeric diazocanes that have been characterized by X-ray crystallography (Scheme 11).
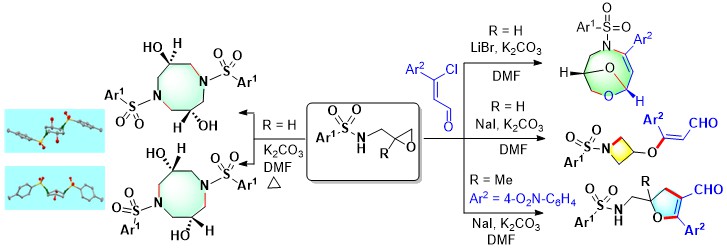
Scheme 11
- Palladium-catalyzed and base-dependent annulation strategies involving N-indolyl-substituted aryl-sulfonamides for the rapid construction of 2-aryl indole and indole-fused six-membered sultams are described The Pd(OAc) 2/Ph3P/Et3N combination delivers indolyl C2 arylated motifs but the Pd(OAc) 2/Ph3P/K2CO3 combination induced intramolecular-Heck cross-coupling affords sultams (Scheme 12).
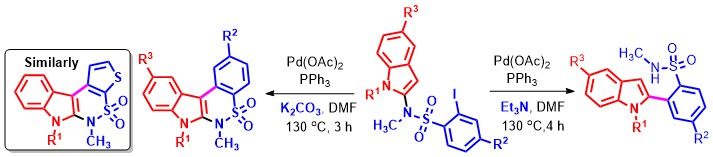
Scheme 12
- Rh(III)-catalyzed vinyl sulfonylation of indoles with ethenesulfonyl fluoride (ESF) has been developed. This chemoselective strategy delivers the corresponding cross-dehydrogenative coupled products in high yields with good functional group tolerance. The cross-coupled adducts could be utilized in click chemistry to access triazoles via thermal (3 + 2) cycloaddition with excellent regioselectivity under metal-free conditions (Scheme 13).
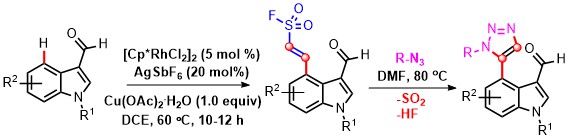
Scheme 13
- Ru(II)-catalyzed oxidative (4+2) annulation of chromene and coumarin carboxylic acids with alkynes gives pyrano-chromones via vinylic C(sp2)-H bond activation; the use of methyl tethered-propargylic alcohols instead of alkynes affords Ru(0)-metal arene-complexes (Scheme 14).
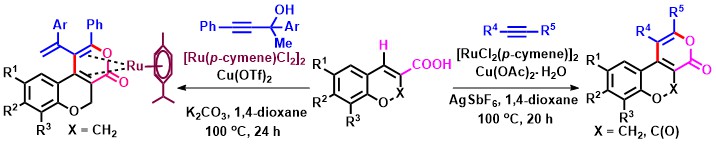
Scheme 14
- Lewis base mediated (3 + 3) annulations of β′/δ-acetoxy allenoates with iminoindolines afford α-carbolines depending on the minor changes in the reaction conditions. The phosphine-catalyzed annulation of δ-acetoxy allenoates with iminoindolines delivers β-H and γ-tosyl containing α-carbolines. Removal of the –CH2CO2Et moiety (by Cα-Cβ bond cleavage) takes place in this reaction. Use of DBU as the catalysis offers α-carbolines that retain –CH2CO2Et moiety but the –Ts group is eliminated. The reaction of β′-acetoxy allenoate with iminoindolines using DABCO affords tetrahydro-α-carbolines but DBU gives α-carbolines different from those using DABCO (Scheme 15).
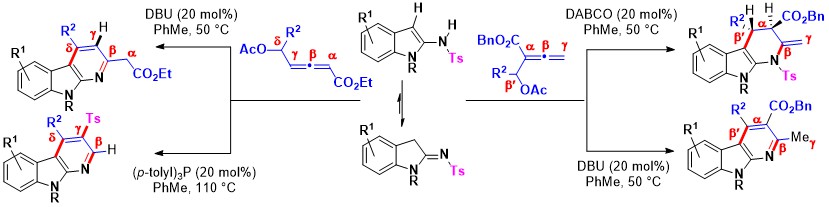
Scheme 15
- DBU-catalyzed spiro-annulation along with ring expansion/ domino reaction of δ-acetoxy allenoates with ene-N-sulfonyl hydrazides afford ring expanded products. By contrast, cycl-3-ene/ane-N-sulfonyl hydrazones under similar conditions deliver pyrazole cores with the same allenoate; the reaction involves a spirocyclic intermediate and a dienyl-amine intermediates. This work has been extended to include (R)-(-)-carvone-derived sulfonyl hydrazide which gave ring-expanded products (Scheme 16).
Scheme 16
- DMAP-catalyzed benzannulation/ lactonization in which δ-acetoxy allenoate functions as a 5C-synthon is explored. This reaction furnishes π-extended coumarins via allylic elimination followed by Mannich-coupling, proton shifts, C-N bond cleavage, and lactonization. The key step for this process is the formation of diene-ammonium intermediate and O-S bond cleavage (Scheme 17).
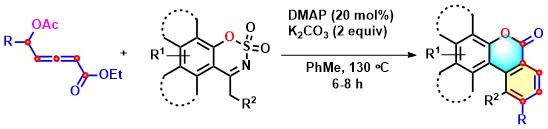
Scheme 17
- DBU catalyzed (3 + 3) annulation of δ-acetoxy allenoates with N-Boc-oxindole, benzofuranone, or pyrazolone gives fused-pyrans while the analogous reaction using DMAP affords dihydropyrans (Scheme 18a). This aspect could be extended to have base-dependent synthesis of tetrasubstituted pyrans or 3,4-dihydropyrans using δ-acetoxy allenoates and enolizable carbonyls like cyclohexan-1,3-dione and ethyl benzoyl. However, the reaction of δ-acetoxy allenoates with ethyl benzoyl acetate mediated by KOtBu affords diethyl 3-vinylpent-2-enedioates (Scheme 18b).
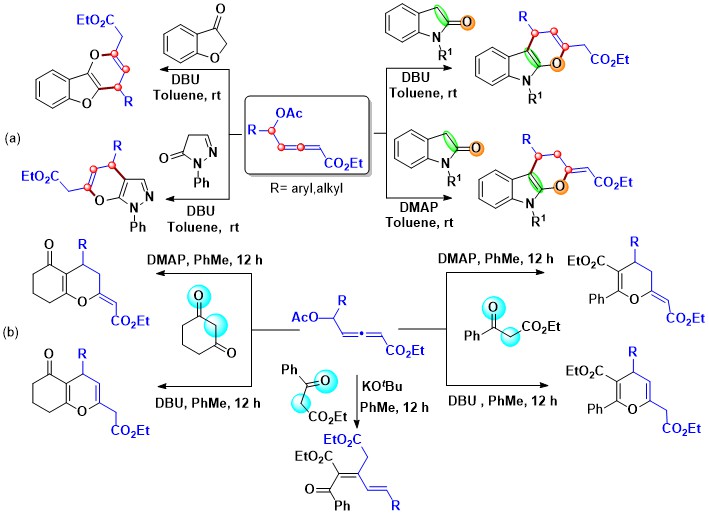
Scheme 18
- Lewis base dependent (3 + 3) and (4 + 2) annulations of β′-acetoxy allenoates with N-sulfonyl ketimines offer m-teraryl and fused dihydropyridines depending on the tertiary amine. The triazabicyclodecene (TBD)-catalyzed (3 + 3) delivers fused 1,4-hydropyridines. The same reactants under DMAP catalysis offer m-teraryl scaffolds (Scheme 19).
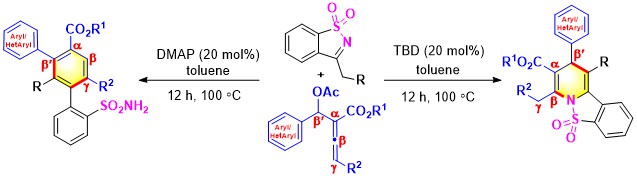
Scheme 19
- Ru-catalyzed oxidative (4+2) annulation of chromene and coumarin carboxylic acids with alkynes gives pyrano-chromones as expected. However, the use of methyl tethered-propargylic alcohols instead of alkynes affords stable Ru(0) complexes.19 In a somewhat related work on propargylic alcohols, Cu(II)-catalyzed decarboxylative oxidative (4+2) annulation of coumarin-3-carboxylic acids with tert-propargylic alcohols involving indirect C-H functionalization leads to diverse naphthochromenones (Scheme 20).
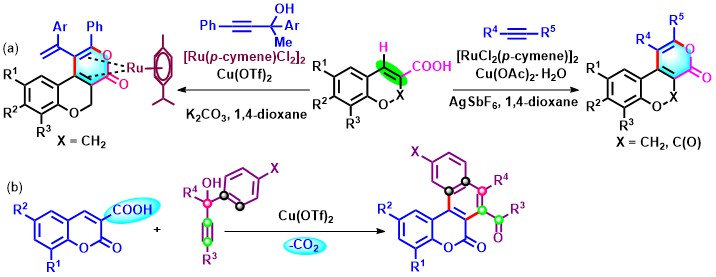
Scheme 20
- Two distinct pathways involving tertiary amine-controlled chemo-, regio-, and stereo-selective carbo-annulation
between δ-acetoxy allenoates and isothiazole dioxides with the allenoate acting as a 4C-synthon have been
demonstrated. Thus DABCO prompted diastereoselective spiroannulation offering multiple chiral centered spirocyclic
scaffolds from allenoates has been discovered. By contrast, DMAP catalyzed benzannulation using allenoates leads to
m-teraryls that are substitutionally different from that using phosphine catalysis (Scheme 1).