
Organophosphorus Chemistry Group
Current Research interests
![]() Organophosphorus
Chemistry
Organophosphorus
Chemistry
![]() Allene chemistry
Allene chemistry
![]() Catalytic Organic Transformations
Catalytic Organic Transformations
Research Highlights
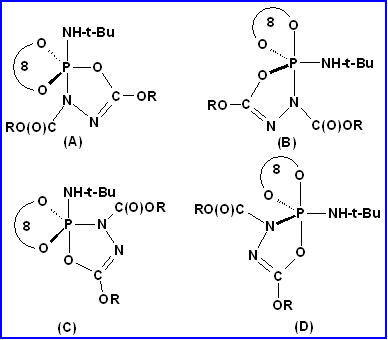
(1) New pentacoordinate phosphorus compounds that throw light on the relative apicophilicities of substituents attached to trigonal bipyramidal phosphorus have been synthesized. These results are in contrast to the apicophilicity order given in standard books on Phosphorus Chemistry. The topic is important in the context of reaction mechanisms at tetracoordinate phosphate esters.
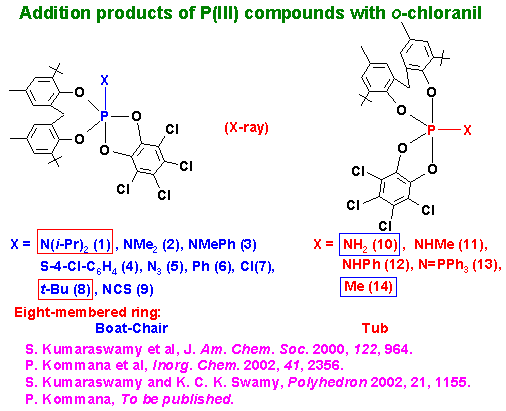
Apicophilicity rules are not followed in many of our compounds shown above.
[cf compounds 1 and 10 and compounds 8 and 14]
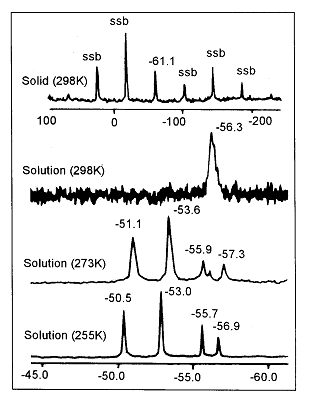
Unusual V.T. 31P NMR [Toluene-d8 solution] is exhibited by some of the above compounds
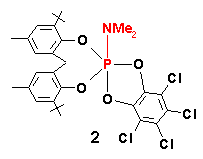
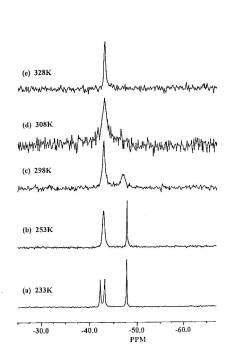
![]()
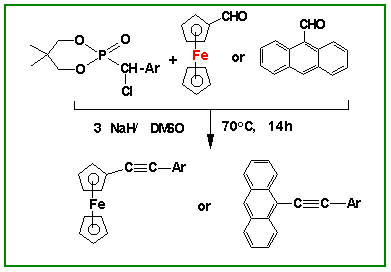
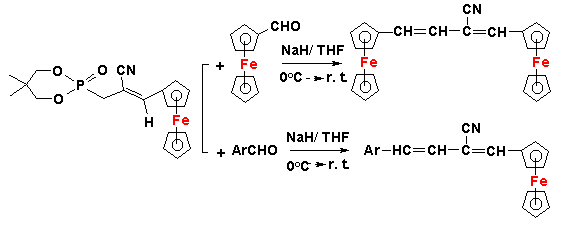
(2) A novel class of compounds (OCH2CMe2CH2O)P(O)CH(X)Ar [X = Cl, Br, I] has been developed and used in organic
synthesis. These compounds have been found to be versatile substrates that can
be adapted to Wadsworth-Emmons reactions. In particular, highly
substituted olefins such as Cl(Ar)C=C(Ar')(Ar") can be readily synthesized
using this route. New
unsymmetrical acetylenes containing ferrocenyl/ anthracenyl residues
have also been prepared. The utility
of the Arbuzov reaction for the facile
synthesis of several hitherto inaccessible alpha-substituted phosphonates has been
developed. A very convenient route to the
biologically important alpha-aminophosphonic
acids [presented in
CRSI-Symposium-III] has been developed. Several phosphonates of
synthetic utility have been prepared using Baylis-Hillman
methodology.
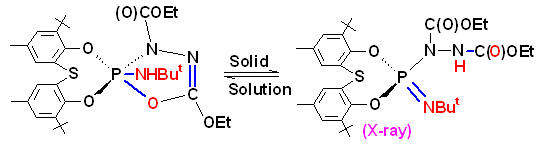
(3) A very unusual 1,3-(P,N) mode of dipolar addition to P(III) azides and isocyanates (completely different from those involving normal organic azides and isocyanates) as well as a unique ring expansion (five to nine membered) of a phosphorus heterocycles have been recently discovered. This chemistry adds a new dimension to the highly useful dipolar addition reactions in organic chemistry.
Cycloaddition and Cyclization Reactions
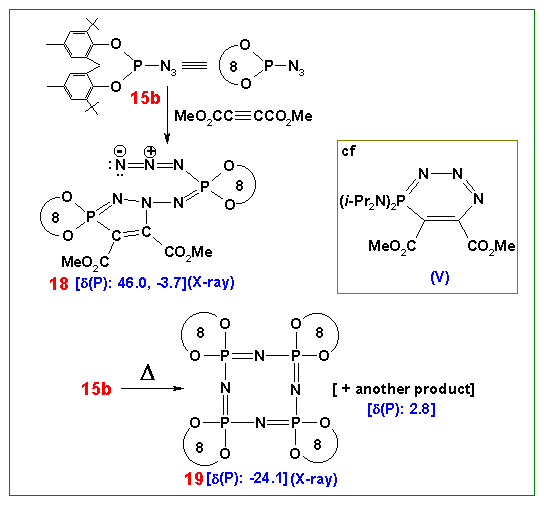
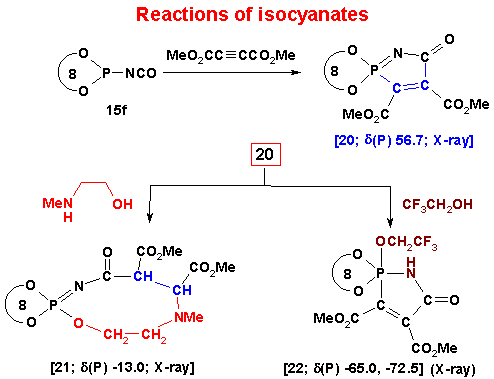
(4) An
elegant and systematic way to construct very short hydrogen bonds utilizing a cyclic phosphate has
been developed. The results have
implications as regards the role of imidazolyl
residues in RNA hydrolysis/ cyclization.
Very Short Hydrogen Bonds
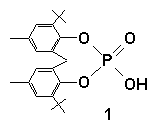
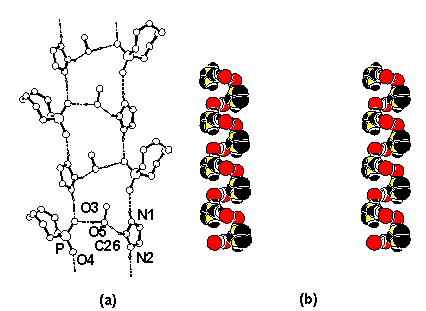
1.Imidazole.MeOH showing Hydrogen bonding
and helical motif {C(26)[-H(26)]…O(5) 3.090(4) Å}
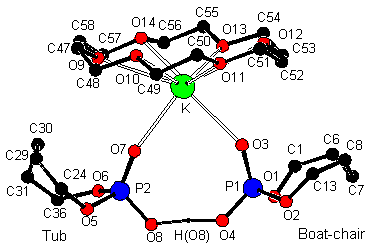
A complex of 1, deprotonated 1 and [K, 18-Crown-6];O(8)[-H(8)]-O(4) 2.397(4) Å
(5) New macrocycles based on a cyclodiphosphazane skeleton have been discovered. Novel P(III) compounds with multi-cyclophosphazane units have been designed for further work.
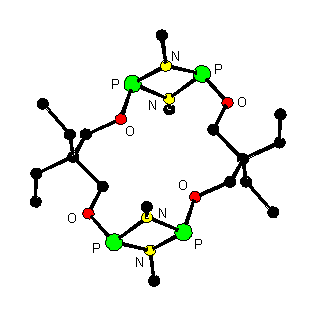
[(OCH2CEt2CH2O){P(N-t-Bu)2P}]2
___________________________________________________________________________________________________________________________________________
Home -
Research Interest- Research Group- Funding- Publications
School of Chemistry -
University of Hyderabad