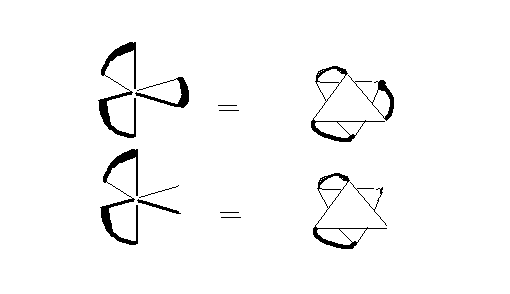
Symmetry and Chirality
An object is said to be chiral when it is not superimposable on its mirror image. 'Chirality' is derived from the Greek word for hand. A chiral molecule is distinguishable from its mirror image just like the left hand is distinguishable from the right hand. The two isomers related this way are called enantiomers.
Molecules which do not possess any symmetry elements other than the C1 axis are said to be asymmetric. Molecules which have one or more proper rotation axes(Cn, n > 1), but no improper rotation axes (Sn) are said to be dissymmetric. All asymmetric and dissymmetric molecules will be chiral. In other words, the absence of Sn is the necessary and sufficient condition for chirality. (Note that S1 is the same as a mirror plane, and S2 is the same as the inversion centre.) How do you understand this requirement?
Chirality arises in organic chemistry mainly through asymmetric centres. There are also many examples of compounds which do not belong to this category.
Here are two examples of dissymmetric molecules. The top one is a tris-chelate, M(AA)3, where AA is a bidentate ligand like ethylenediamine. The lower one is cis-M(AA)2X2. Convince yourself that the point groups of these molecules are D3 and C2. What will be the point groups, if instead of AA, the bidentate ligand is AB?
A non-chiral molecule which does not possess a mirror plane or inversion centre is illustrated below:
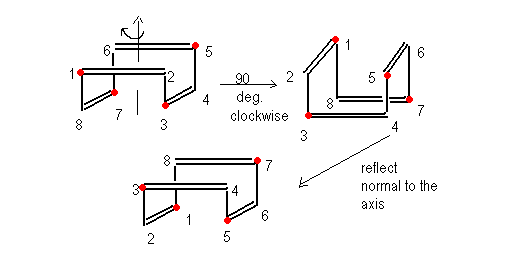
1,3,5,7-tetramethylcycloctatetraene: What improper rotation axis does it have?
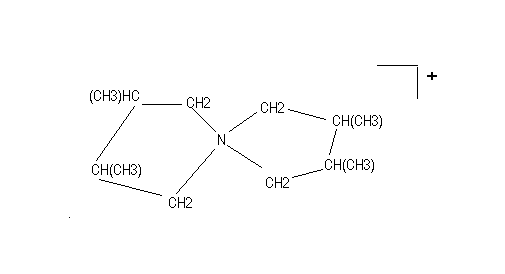
A study problem: Are there asymmetric carbon atoms in the following ion? What are the possible isomeric structures? Which ones are chiral? (see, Shriver, Atkins, Langford p.57)