P
(x)Q
(x)Hermite equation
![]()
![]()
Legendre equation
![]()
![]()
Associated Legendre equation
![]()
![]()
Laguerre equation
![]()
![]()
Associated Laguerre equation
![]()
![]()
Special Functions
An important type of ordinary differential equations is,
y'' + P(x) y' + Q(x) y = 0
|
P (x) |
Q (x) |
|
|
Hermite equation |
|
|
|
Legendre equation |
|
|
|
Associated Legendre equation |
|
|
|
Laguerre equation |
|
|
|
Associated Laguerre equation |
|
|
Under certain conditions, the above equations have polynomial solutions.
Hermite polynomials : a = n, an integer >/= 0
|
n |
Hn(x) |
|
0 |
1 |
|
1 |
2x |
|
2 |
4x2 - 2 |
|
3 |
8x3- 12x |
Hn+1(x) = 2xHn(x) - 2nHn-1(x)
If the Hermite equation is modified with P(x) = 0, and Q(x) = 1 - x2 + 2n, there results the Hermite-Gauss equation. Its solutions are ![]() . When normalised by multiplying with
. When normalised by multiplying with ![]() these become the wave functions of the harmonic oscillator.
these become the wave functions of the harmonic oscillator.
Legendre polynomials : x in the range -1 to 1 and l, an integer > = 0
|
l |
Pl(x) |
|
0 |
1 |
|
1 |
x |
|
2 |
(1/2)(3x2 -1) |
|
3 |
(1/2)(5x3-3x) |
Pl
+1(x) = [x(2l + 1)Pl(x) - lPl-1(x)] / (l + 1)
Associated Legendre polynomials : integer m < = l
|
l |
m |
|
|
0 |
0 |
1 |
|
1 |
0 |
x |
|
1 |
1 |
(1 - x2)1/2 |
|
2 |
0 |
(1/2)(3x2 - 1) |
|
2 |
1 |
3x(1 - x2)1/2 |
|
2 |
2 |
3(1 - x2) |
![]()
Spherical harmonics :
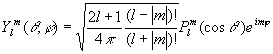
These are the wave functions of the rigid rotor. They also serve as the angular part of the hydrogen atom wave functions.
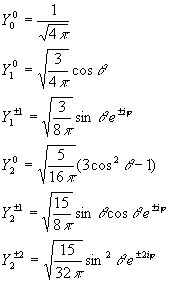
|
|
1 - x |
|
|
x2 - 4x + 2 |
|
|
-x3 + 9x2 - 18x + 6 |
|
|
x4 - 16x3 + 72x2 - 96x + 24 |
|
|
-x5 + 25x4 - 200x3 + 600x2 - 600x + 120 |
Associated Laguerre polynomials : integer k > = 0
![]()
The radial part of the hydrogen atom wave functions are obtained as,
 , where n is the principal quantum number and r is proportional to the radial coordinate of the electron ( r = 2r/nao).
, where n is the principal quantum number and r is proportional to the radial coordinate of the electron ( r = 2r/nao).

------------------------------------------
References : Eyring, Walter and Kimball "Quantum Chemistry"
Margenau and Murphy "Mathematics of Physics and Chemistry"