|
Inorganic chemistry/Materials/Polymers/Catalysis/High-Energy materials
Our group s research focuses on health, energy, the environment, and defence (HEED). The primary aim of our study is to design materials from main group elements that address fundamental issues related to the efficient use of molecular materials in energy and high-energy applications (propellants and explosives). We have been actively involved in producing nanomaterials, small molecules, and polymer materials, as well as conducting application studies in these areas . Research highlights High-energy materials for spontaneous and controlled energy release in propellents Nitrogen and boron-rich small molecules and salts as high-energy materials Aluminium nanopartclces for propellents Methodology development for the synthesis of metal and metal chalcogenide nanoparticles Production of organic surfactant-free nanoparticles for optoelectronic and biological applications Catalysis by nanoparticles (reaction catalysis and degradation catalysis) Metal complexes and their catalytic activities Synthesis of cyclic and polymeric carbonates from carbon dioxide Polymers having phosphorus, polycarbonates and polyethers with an intrinsic disordered framework for Li-ion batteries and flame-retardant material.
High-energy materials We have synthesized boron-rich hybrid inorganic-organic salts using closo-dodecaborate [(B12H12)]2- as an anion, imidazolium, triazolium, and tetrazolium, and other nitrogen-rich cations. Additionally, we created a variety of nitrogen-rich molecules derived from triazoles and tetrazoles, studying their energetic properties.
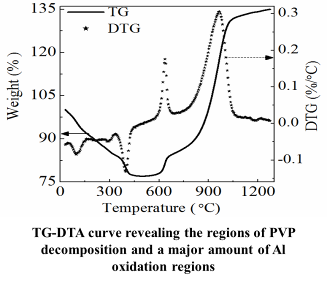
We have developed a chemical synthesis method for producing polymer-stabilized aluminum nanoparticles for use in propellants. Our research has shown that the solution-phase synthetic method is effective in obtaining aluminum nanoparticles stabilized within matrices of poly(vinylpyrrolidone), poly(methyl methacrylate), dextrin, and other plasticizers. Moreover, these polymer-stabilized nanoparticles have demonstrated stability without oxidation for at least ten months when exposed to an open atmosphere.
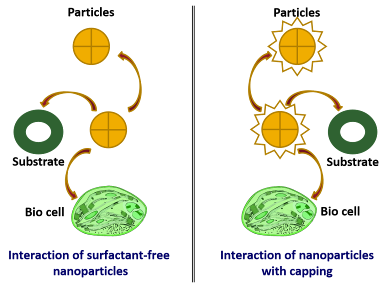
Nanomaterials Nanoparticles produced through synthetic chemical methods are typically surrounded by organic surfactant molecules or polymers, known as capping or stabilizing agents. Unfortunately, these capping agents can inhibit the ability to utilize the surface or size-dependent properties of the nanoparticles fully. The reasons for this limitation are as follows. (1) Surrounding of chemically synthesized nanoparticles by organic surfactant molecules or any other surface coating would affect the processes such as recombination or charge transfer, (2) The presence of organic surfactants around nanoparticles hinders the mobility of charge carriers in electronic devices, (3) These molecules shield the active sites of the catalyst causing less catalytic activity than its potential, and (4) since they are not detached from particles they may be toxic when used in biomedical applications.
Schematic representation of chemically synthesized nanoparticles surrounded by organic surfactant molecules and their interactions.
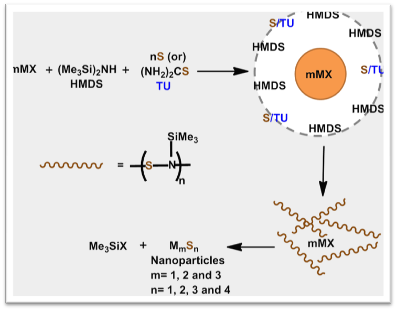
However, we have developed a novel HMDS-assisted methodology for synthesizing metal sulfide and selenide nanoparticles (CuS, Cu2S, CuInS2, CuInSe2, AgS, CdS, PbS, PbSe, and BiS), which are free from any organic impurities. These materials have enhanced efficiency in various fields.
Schematic representation of HMDS-assisted synthesis of metal chalcogenides
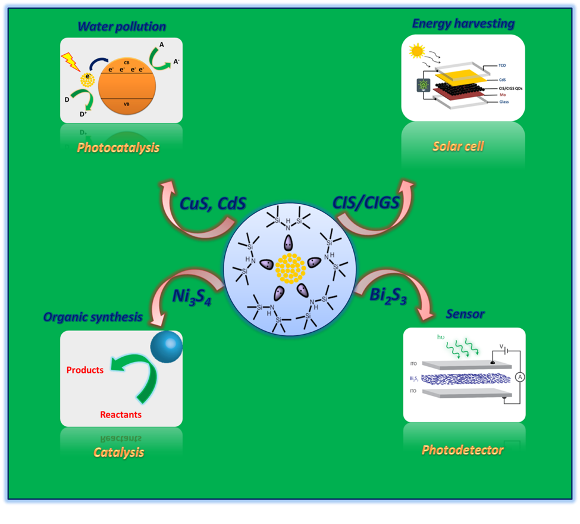
Some nanoparticles without surfactants have been used as catalysts in organic functional group transformations, in the degradation of organic dyes, and as photocatalysts for water purification.
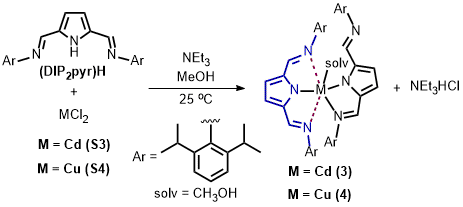
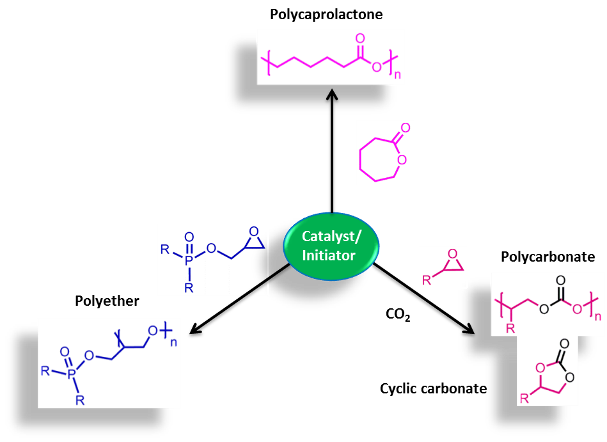
Inorganic complexes - Catalysis We have synthesized 2,5-bis{N-(2,6-diisopropylphenyl)iminomethyl}pyrrolyl complexes of Zn(II), Cd(II) and Cu(II) and studied their catalytic activities in carbon dioxide fixation. Using these complexes as a catalyst, a variety of cyclic carboantes and phosphorous-containing polycarbonates have been synthesized.
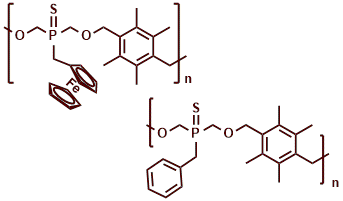
Polymers We have designed polyethers utilizing bis(hydroxymethyl)phosphine sulfides, which feature an intrinsically disordered framework that enhances lithium-ion (Li-ion) conductivity. One of our solid polymer electrolytes achieved an impressive conductivity of 1.4 x 10-⁴ S cm- at room temperature. Furthermore, the incorporation of phosphorus into these polymers is anticipated to provide flame-retardant properties to the electrolyte. These findings are highly beneficial for improving the efficiency of Li-ion batteries.
|

|
|